Connecting Innovation to Purpose
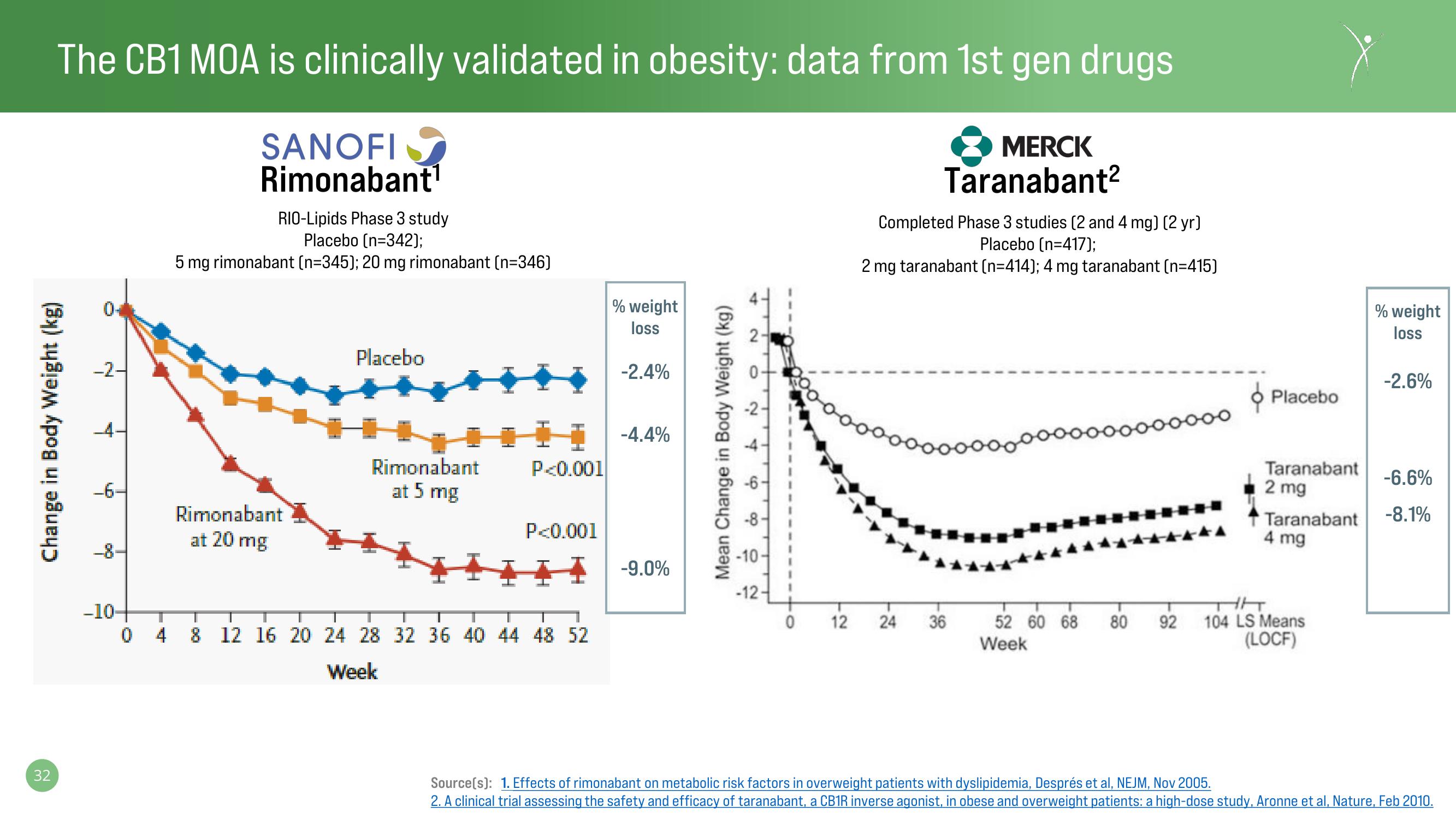
The CB1 MOA is clinically validated in obesity: data from 1st gen drugs
MERCK
Taranabant²
Change in Body Weight (kg)
32
-6-
-10-
||
SANOFI
Rimonabant¹
RIO-Lipids Phase 3 study
Placebo (n=342);
5 mg rimonabant (n=345); 20 mg rimonabant (n=346)
Rimonabant
at 20 mg
Placebo
+
Rimonabant
at 5 mg
KH
P<0.001
P<0.001
H
8 12 16 20 24 28 32 36 40 44 48 52
Week
% weight
loss
-2.4%
-4.4%
-9.0%
2
Mean Change in Body Weight (kg)
प
-12-
0
12
Completed Phase 3 studies (2 and 4 mg) (2 yr)
Placebo (n=417);
2 mg taranabant (n=414); 4 mg taranabant (n=415)
24
oooo
36
oooooooooooo
52 60 68
Week
80
92
Placebo
Taranabant
2 mg
Taranabant
4 mg
104 LS Means
(LOCF)
% weight
loss
-2.6%
-6.6%
-8.1%
Source(s): 1. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia, Després et al, NEJM, Nov 2005.
2. A clinical trial assessing the safety and efficacy of taranabant, a CB1R inverse agonist, in obese and overweight patients: a high-dose study, Aronne et al, Nature, Feb 2010.View entire presentation