BioNTech Investor Day Presentation Deck
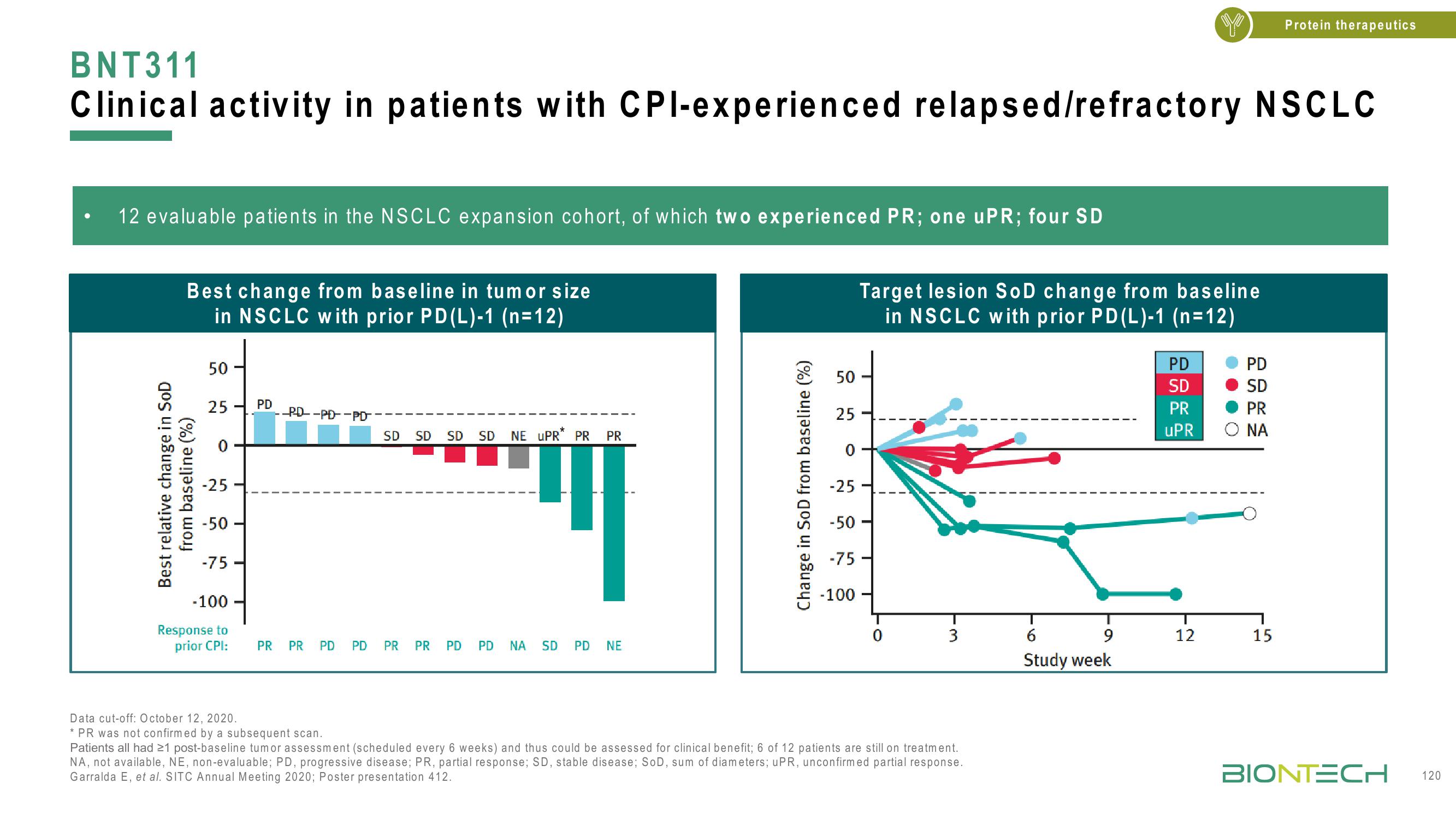
12 evaluable patients in the NSCLC expansion cohort, of which two experienced PR; one uPR; four SD
BNT311
Clinical activity in patients with CPI-experienced relapsed/refractory NSCLC
Best change from baseline in tumor size
in NSCLC with prior PD(L)-1 (n=12)
Best relative change in SoD
from baseline (%)
50
25. PD
-25
-50
-75
-100
Response to
prior CPI:
--PD-PD-PD
SD SD SD
SD
NE UPR* PR PR
PR PR PD PD PR PR PD PD ΝΑ SD PD
NE
Change in SoD from baseline (%)
50
25
0
-25
-50
-75
-100
Target lesion SoD change from baseline
in NSCLC with prior PD (L)-1 (n=12)
3
Data cut-off: October 12, 2020.
*PR was not confirmed by a subsequent scan.
Patients all had 21 post-baseline tumor assessment (scheduled every 6 weeks) and thus could be assessed for clinical benefit; 6 of 12 patients are still on treatment.
NA, not available, NE, non-evaluable; PD, progressive disease; PR, partial response; SD, stable disease; SoD, sum of diameters; uPR, unconfirmed partial response.
Garralda E, et al. SITC Annual Meeting 2020; Poster presentation 412.
9
6
Study week
Y
PD
SD
PR
uPR
12
PD
SD
PR
ΝΑ
Protein therapeutics
15
BIONTECH
120View entire presentation