23andMe Investor Day Presentation Deck
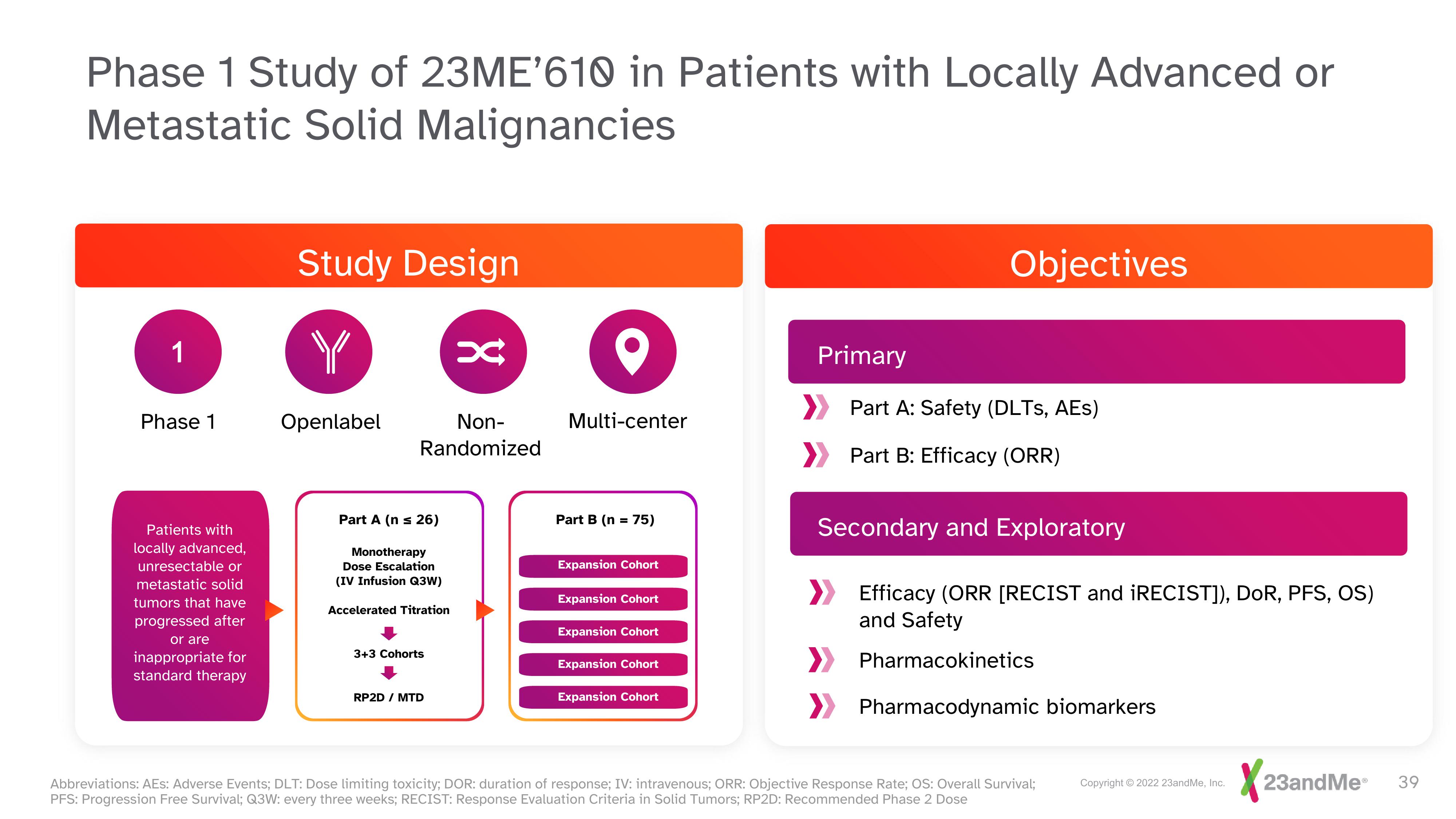
Phase 1 Study of 23ME'610 in Patients with Locally Advanced or
Metastatic Solid Malignancies
1
Phase 1
Patients with
locally advanced,
unresectable or
metastatic solid
tumors that have
progressed after
or are
inappropriate for
standard therapy
Study Design
Y
Openlabel
Non-
Randomized
Part A (n = 26)
Monotherapy
Dose Escalation
(IV Infusion Q3W)
Accelerated Titration
3+3 Cohorts
x
RP2D / MTD
Multi-center
Part B (n = 75)
Expansion Cohort
Expansion Cohort
Expansion Cohort
Expansion Cohort
Expansion Cohort
Primary
Objectives
Part A: Safety (DLTS, AES)
Part B: Efficacy (ORR)
Secondary and Exploratory
Efficacy (ORR [RECIST and iRECIST]), DOR, PFS, OS)
and Safety
Pharmacokinetics
Pharmacodynamic biomarkers
Abbreviations: AEs: Adverse Events; DLT: Dose limiting toxicity; DOR: duration of response; IV: intravenous; ORR: Objective Response Rate; OS: Overall Survival;
PFS: Progression Free Survival; Q3W: every three weeks; RECIST: Response Evaluation Criteria in Solid Tumors; RP2D: Recommended Phase 2 Dose
Copyright © 2022 23and Me, Inc.
23andMe® 39View entire presentation