BioAtla Investor Presentation Deck
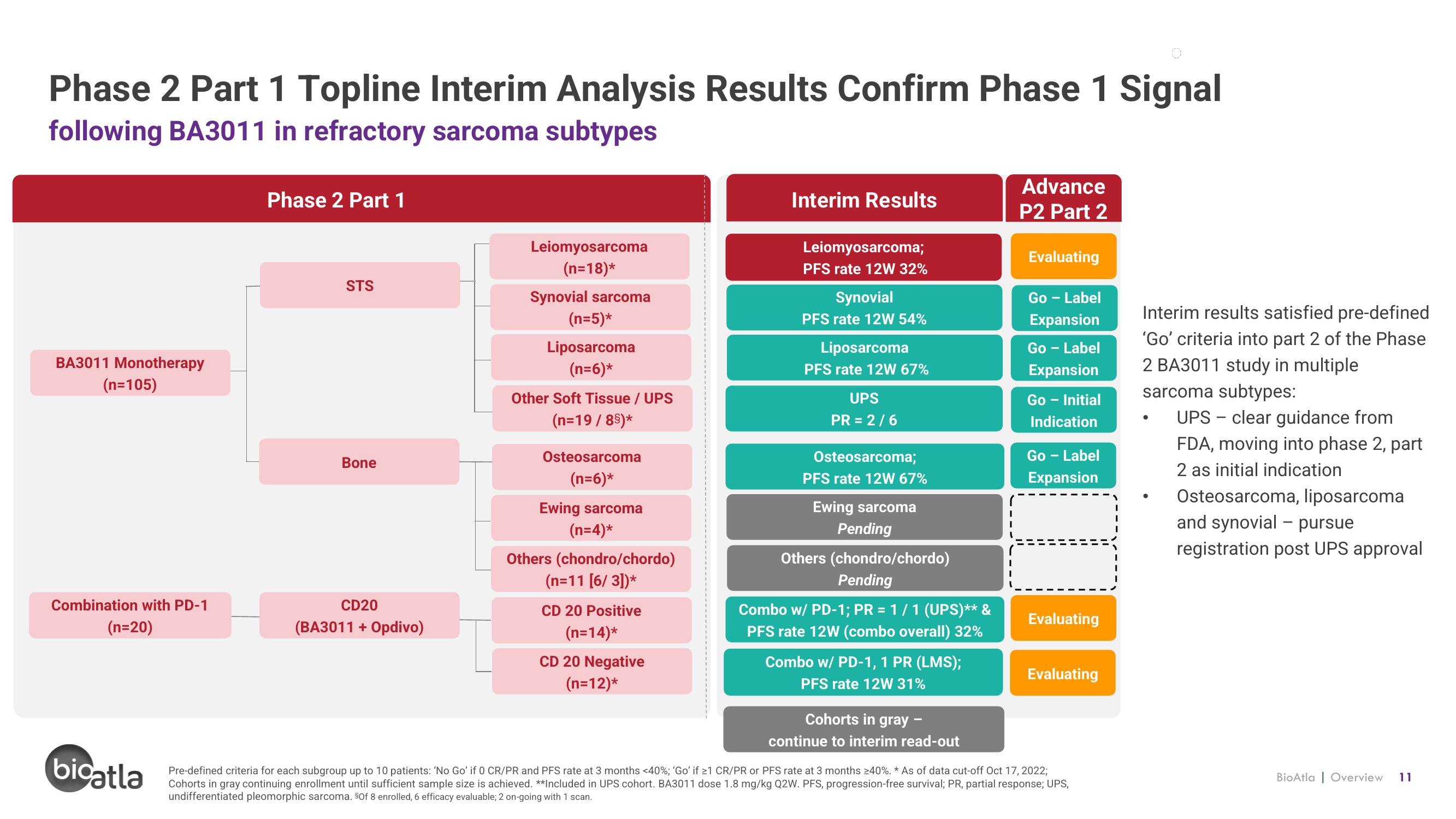
Phase 2 Part 1 Topline Interim Analysis Results Confirm Phase 1 Signal
following BA3011 in refractory sarcoma subtypes
BA3011 Monotherapy
(n=105)
Combination with PD-1
(n=20)
bicatla
Phase 2 Part 1
STS
Bone
CD20
(BA3011 + Opdivo)
Leiomyosarcoma
(n=18)*
Synovial sarcoma
(n=5)*
Liposarcoma
(n=6)*
Other Soft Tissue / UPS
(n=19 / 85)*
Osteosarcoma
(n=6)*
Ewing sarcoma
(n=4)*
Others (chondro/chordo)
(n=11 [6/ 3])*
CD 20 Positive
(n=14)*
CD 20 Negative
(n=12)*
Interim Results
Leiomyosarcoma;
PFS rate 12W 32%
Synovial
PFS rate 12W 54%
Liposarcoma
PFS rate 12W 67%
UPS
PR = 2/6
Osteosarcoma;
PFS rate 12W 67%
Ewing sarcoma
Pending
Others (chondro/chordo)
Pending
Combo w/ PD-1; PR = 1/1 (UPS)** &
PFS rate 12W (combo overall) 32%
Combo w/ PD-1, 1 PR (LMS);
PFS rate 12W 31%
Cohorts in gray
continue to interim read-out
Advance
P2 Part 2
Evaluating
Go - Label
Expansion
Go - Label
Expansion
Go - Initial
Indication
Go - Label
T
Expansion
Evaluating
Evaluating
Pre-defined criteria for each subgroup up to 10 patients: 'No Go' if 0 CR/PR and PFS rate at 3 months <40%; 'Go' if ≥1 CR/PR or PFS rate at 3 months ≥40%. * As of data cut-off Oct 17, 2022;
Cohorts in gray continuing enrollment until sufficient sample size is achieved. **Included in UPS cohort. BA3011 dose 1.8 mg/kg Q2W. PFS, progression-free survival; PR, partial response; UPS,
undifferentiated pleomorphic sarcoma. §Of 8 enrolled, 6 efficacy evaluable; 2 on-going with 1 scan.
Interim results satisfied pre-defined
'Go' criteria into part 2 of the Phase
2 BA3011 study in multiple
sarcoma subtypes:
UPS - clear guidance from
FDA, moving into phase 2, part
2 as initial indication
Osteosarcoma, liposarcoma
and synovial - pursue
registration post UPS approval
BioAtla| Overview 11View entire presentation