AstraZeneca Results Presentation Deck
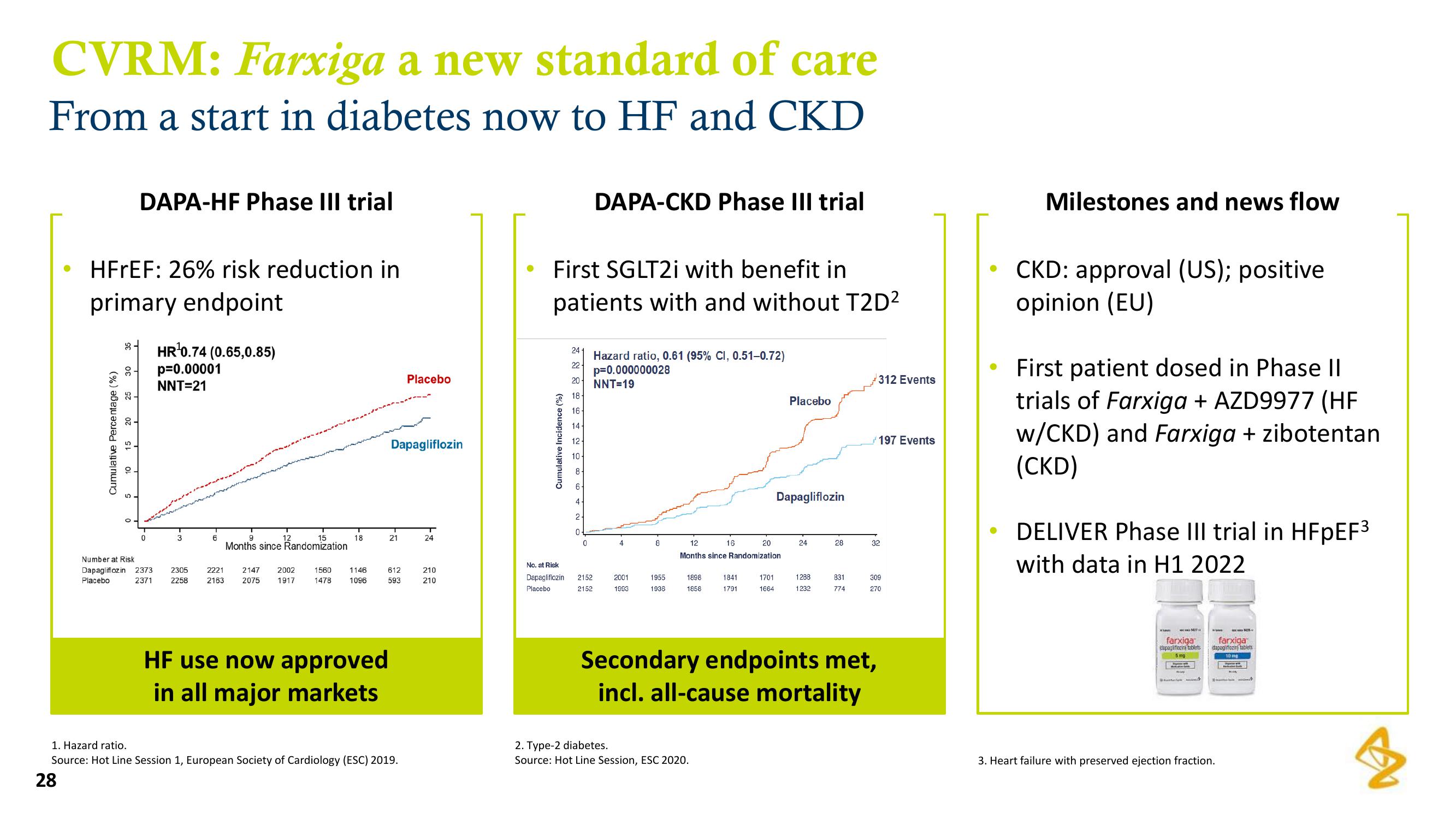
CVRM: Farxiga a new standard of care
From a start in diabetes now to HF and CKD
HFrEF: 26% risk reduction in
primary endpoint
Cumulative Percentage (%)
DAPA-HF Phase III trial
3-
HR¹0.74 (0.65,0.85)
p=0.00001
NNT=21
Number at Risk
Dapagliflozin 2373 2305
2371
Placebo
2258
2221
2163
9
15
Months since Randomization
2147
2075
12
2002
1917
1560
1478
18
1146
1096
HF use now approved
in all major markets
Dapagliflozin
21
612
593
Placebo
1. Hazard ratio.
Source: Hot Line Session 1, European Society of Cardiology (ESC) 2019.
28
24
210
210
[.
First SGLT2i with benefit in
patients with and without T2D²
Cumulative Incidence (%)
No. at Risk
Dapagliflozin
Placebo
24
22
20
18
16-
14
12
10
8
6
4
2
0
0
DAPA-CKD Phase III trial
Hazard ratio, 0.61 (95% CI, 0.51-0.72)
p=0.000000028
NNT=19
2152
2152
2001
1993
8
1955
1936
1898
1858
12
Months since Randomization
16
2. Type-2 diabetes.
Source: Hot Line Session, ESC 2020.
20
1841
1791
1701
1664
Placebo
Dapagliflozin
24
1288
1232
28
831
774
312 Events
Secondary endpoints met,
incl. all-cause mortality
197 Events
32
309
270
Milestones and news flow
CKD: approval (US); positive
opinion (EU)
First patient dosed in Phase II
trials of Farxiga + AZD9977 (HF
w/CKD) and Farxiga + zibotentan
(CKD)
DELIVER Phase III trial in HFpEF³
with data in H1 2022
MYA
1077
farxiga
dapagliflozin tablets
5 mg
www HW-
farxiga
dapaglificen tablets
10 mg
3. Heart failure with preserved ejection fraction.
24
3View entire presentation