AstraZeneca Investor Day Presentation Deck
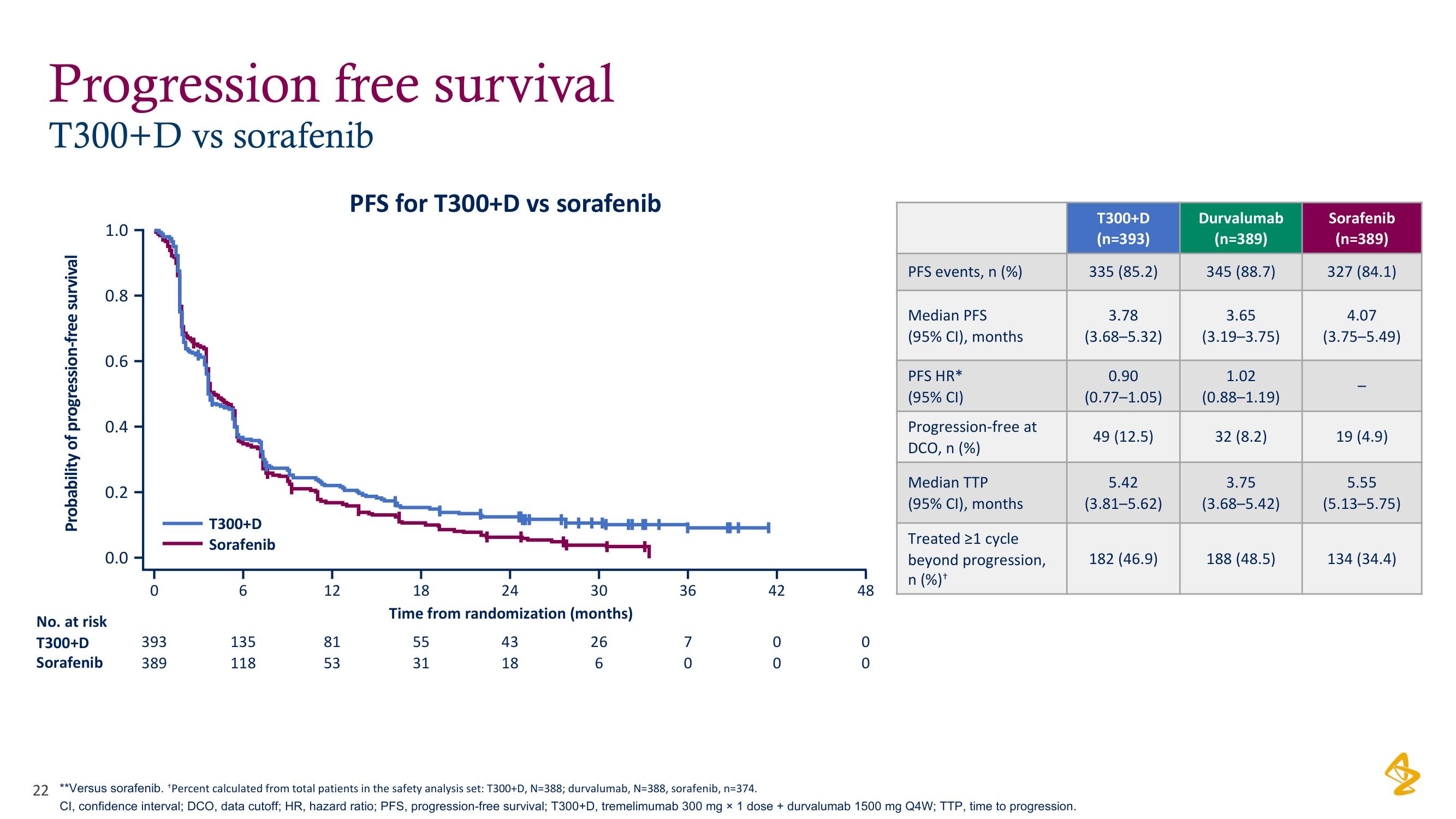
Progression free survival
T300+D vs sorafenib
Probability of progression-free survival
1.0
0.8
0.6-
0.4
0.2-
0.0
No. at risk
T300+D
Sorafenib
0
393
389
T300+D
Sorafenib
6
135
118
12
81
53
PFS for T300+D vs sorafenib
18
24
30
Time from randomization (months)
55
31
43
18
26
6
36
7
0
H
42
0
0
48
0
0
PFS events, n (%)
Median PFS
(95% CI), months
PFS HR*
(95% CI)
Progression-free at
DCO, n (%)
Median TTP
(95% CI), months
Treated 21 cycle
beyond progression,
n (%) +
22 **Versus sorafenib. *Percent calculated from total patients in the safety analysis set: T300+D, N=388; durvalumab, N=388, sorafenib, n=374.
CI, confidence interval; DCO, data cutoff; HR, hazard ratio; PFS, progression-free survival; T300+D, tremelimumab 300 mg x 1 dose + durvalumab 1500 mg Q4W; TTP, time to progression.
T300+D
(n=393)
335 (85.2)
3.78
(3.68-5.32)
0.90
(0.77-1.05)
49 (12.5)
5.42
(3.81-5.62)
182 (46.9)
Durvalumab
(n=389)
345 (88.7)
3.65
(3.19-3.75)
1.02
(0.88-1.19)
32 (8.2)
3.75
(3.68-5.42)
188 (48.5)
Sorafenib
(n=389)
327 (84.1)
4.07
(3.75-5.49)
19 (4.9)
5.55
(5.13-5.75)
134 (34.4)
BView entire presentation