Company Overview
For personal use only
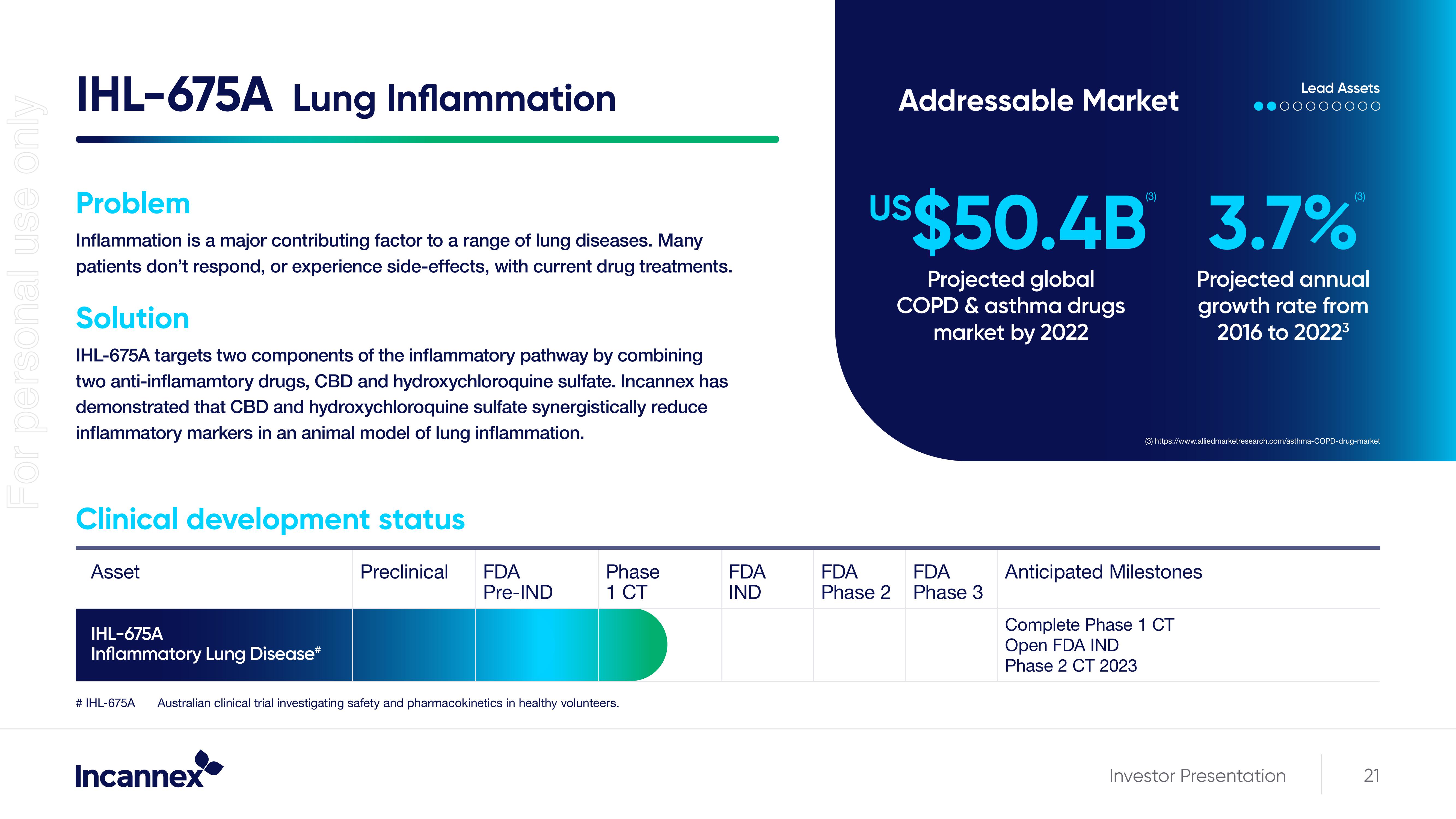
IHL-675A Lung Inflammation
Problem
Inflammation is a major contributing factor to a range of lung diseases. Many
patients don't respond, or experience side-effects, with current drug treatments.
Solution
IHL-675A targets two components of the inflammatory pathway by combining
two anti-inflamamtory drugs, CBD and hydroxychloroquine sulfate. Incannex has
demonstrated that CBD and hydroxychloroquine sulfate synergistically reduce
inflammatory markers in an animal model of lung inflammation.
Clinical development status
Asset
IHL-675A
Inflammatory Lung Disease*
Preclinical FDA
Incannex
Pre-IND
Phase
1 CT
# IHL-675A Australian clinical trial investigating safety and pharmacokinetics in healthy volunteers.
FDA
IND
Addressable Market
FDA
Phase 2
US$50.4B 3.7%
Projected global
COPD & asthma drugs
market by 2022
Projected annual
growth rate from
2016 to 2022³
FDA
Phase 3
Lead Assets
..00000000
(3) https://www.alliedmarketresearch.com/asthma-COPD-drug-market
Anticipated Milestones
Complete Phase 1 CT
Open FDA IND
Phase 2 CT 2023
Investor Presentation
21View entire presentation