BioNTech Results Presentation Deck
Autogene cevum eran (BNT122): First Patient Dosed in Phase 2 Clinical Trial in
Adjuvant Colorectal Cancer
Randomized, Phase 2 trial evaluating autogene cevumeran* in the adjuvant treatment of circulating tumor DNA
positive, surgically resected Stage II (high risk)/Stage III colorectal cancer
●
Colorectal cancer is second deadliest cancer worldwide ¹, 5 year OS in regional disease is 71% ²
SoC in Stage II (high risk) and Stage III CRC after removal of the primary tumor and adjuvant chemotherapy is watchful waiting
ctDNA is a marker for minimal residual disease and thus can identify patients at high risk of disease recurrence³,4
In ctDNA-positive, Stage 2 (high risk) and Stage 3 CRC post AdCTx, duration of disease free survival is 6 months5
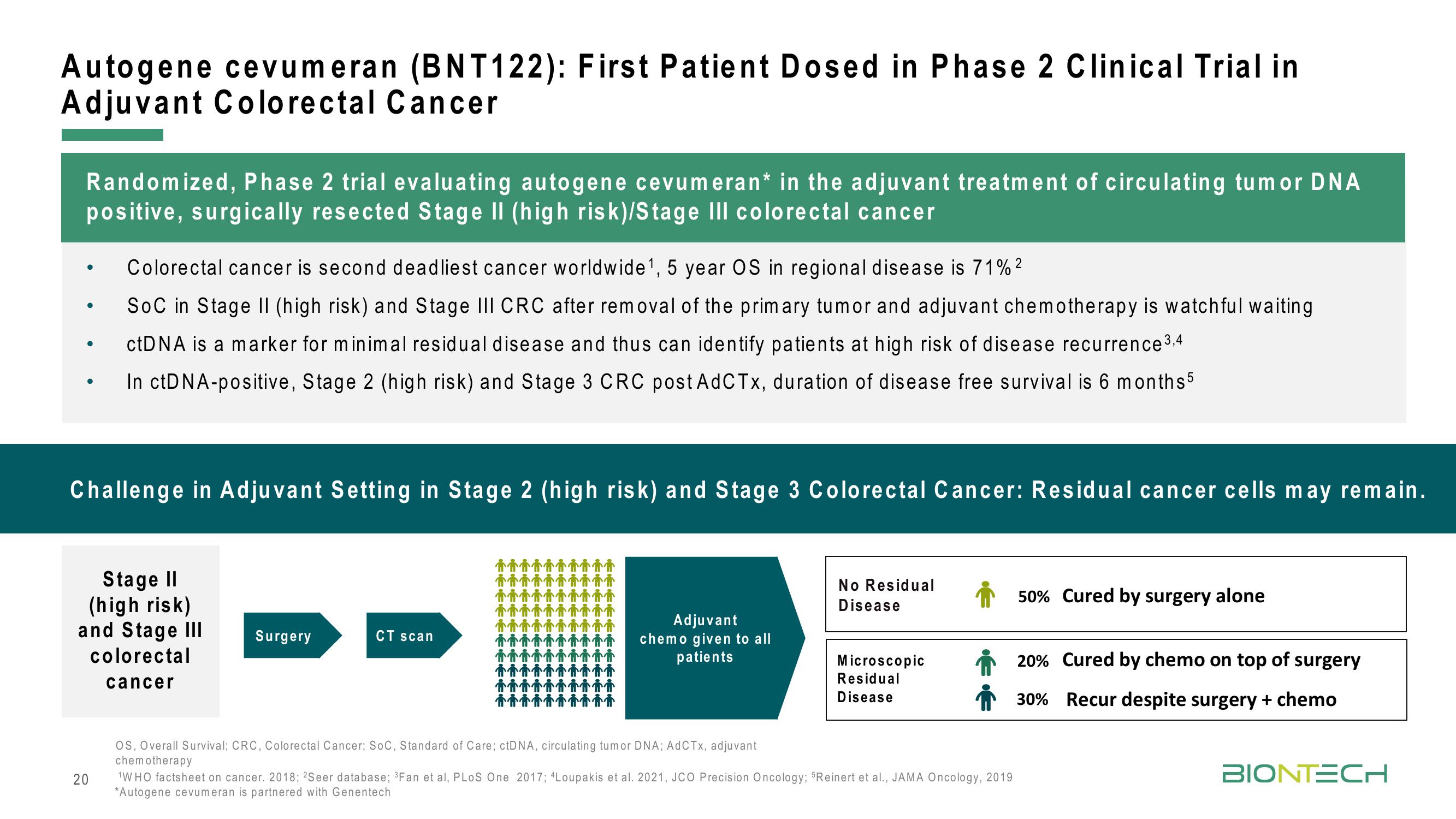
Challenge in Adjuvant Setting in Stage 2 (high risk) and Stage 3 Colorectal Cancer: Residual cancer cells may remain.
Stage II
(high risk)
and Stage III
colorectal
cancer
20
Surgery
CT scan
Adjuvant
chemo given to all
patients
OS, Overall Survival; CRC, Colorectal Cancer; SoC, Standard of Care; ctDNA, circulating tumor DNA; AdCTx, adjuvant
chemotherapy
No Residual
Disease
Microscopic
Residual
Disease
¹WHO factsheet on cancer. 2018; 2Seer database; ³Fan et al, PLoS One 2017; 4Loupakis et al. 2021, JCO Precision Oncology; "Reinert et al., JAMA Oncology, 2019
*Autogene cevumeran is partnered with Genentech
50% Cured by surgery alone
20% Cured by chemo on top of surgery
30% Recur despite surgery + chemo
BIONTECHView entire presentation