Omega Therapeutics IPO Presentation Deck
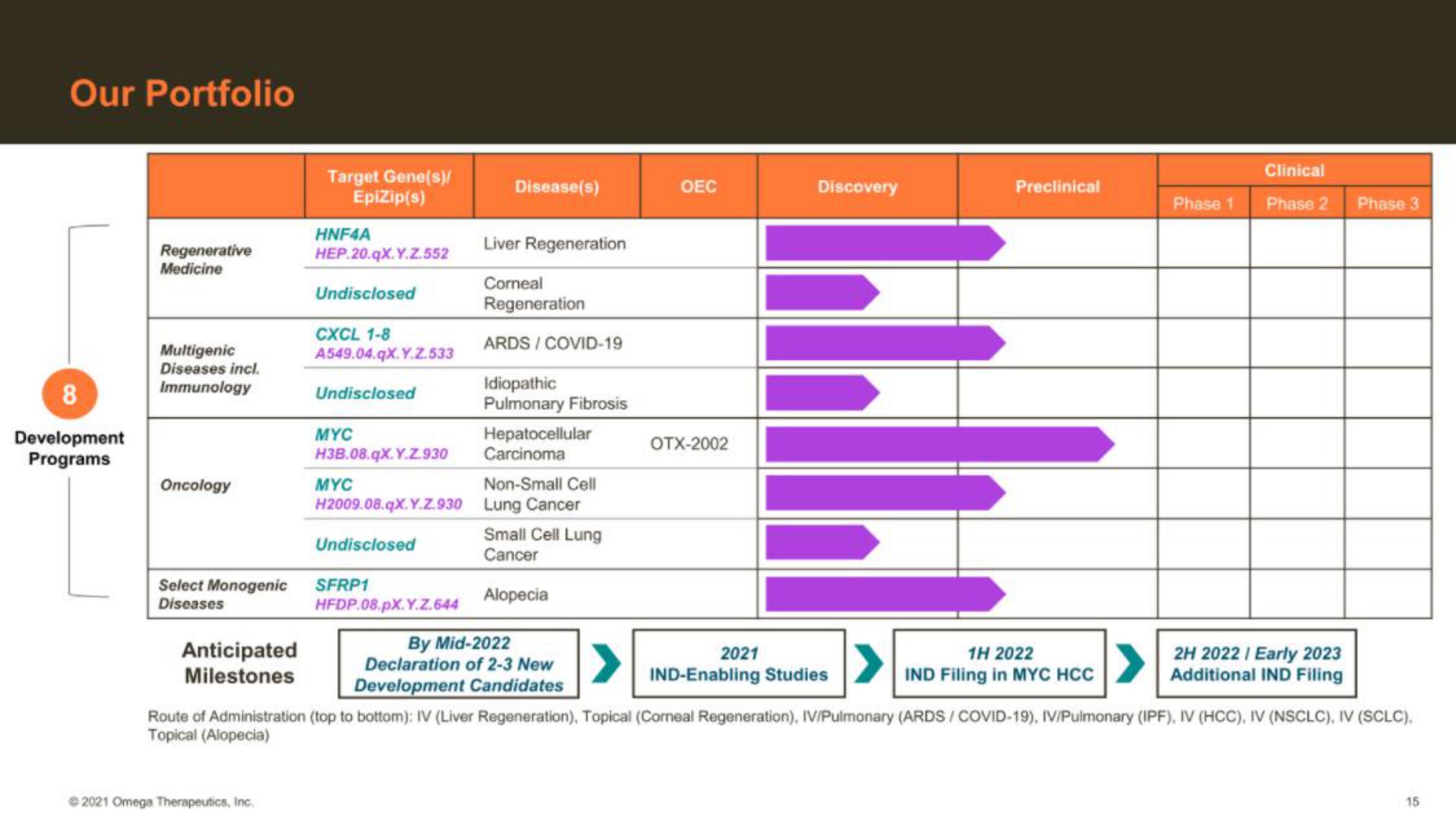
Our Portfolio
8
Development
Programs
Regenerative
Medicine
Multigenic
Diseases incl.
Immunology
Oncology
Select Monogenic
Diseases
Anticipated
Milestones
Target Gene(s)/
EpiZip(s)
©2021 Omega Therapeutics, Inc.
HNF4A
HEP.20.qx.Y.Z.552
Undisclosed
CXCL 1-8
A549.04.qX.Y.Z.533
Undisclosed
MYC
H3B.08.qX.Y.Z.930
MYC
H2009.08.qX.Y.Z.930
Undisclosed
SFRP1
HFDP.08.pX.Y.Z.644
Disease(s)
Liver Regeneration
Corneal
Regeneration
ARDS / COVID-19
Idiopathic
Pulmonary Fibrosis
Hepatocellular
Carcinoma
Non-Small Cell
Lung Cancer
Small Cell Lung
Cancer
Alopecia
By Mid-2022
Declaration of 2-3 New
Development Candidates
OEC
OTX-2002
2021
Discovery
IND-Enabling Studies
Preclinical
1H 2022
IND Filing in MYC HCC
Phase 1
Clinical
Phase 2 Phase 3
2H 2022 | Early 2023
Additional IND Filing
Route of Administration (top to bottom): IV (Liver Regeneration). Topical (Corneal Regeneration), IV/Pulmonary (ARDS/COVID-19), IV/Pulmonary (IPF), IV (HCC), IV (NSCLC), IV (SCLC),
Topical (Alopecia)
15View entire presentation