BenevolentAI Investor Day Presentation Deck
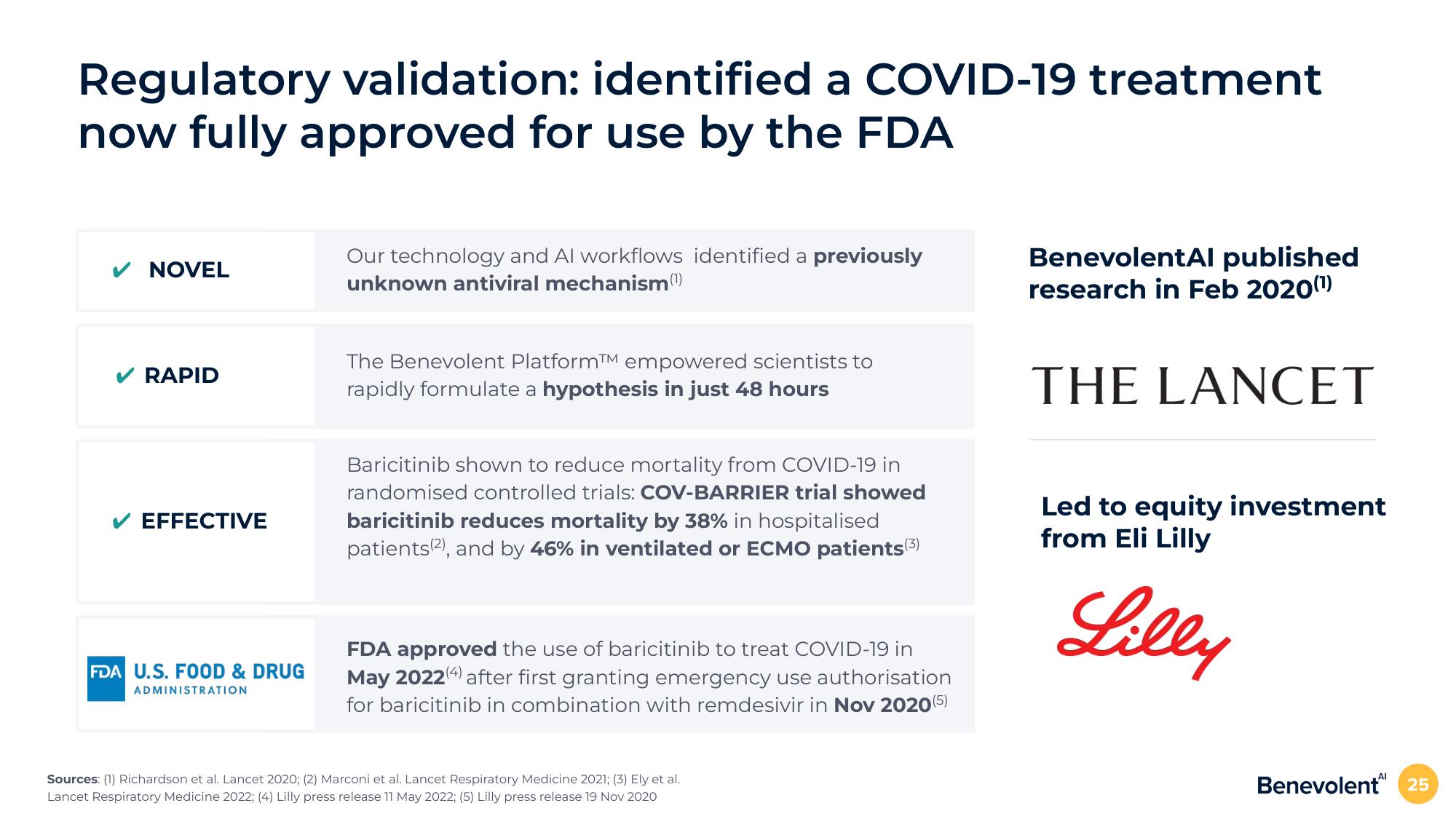
Regulatory validation: identified a COVID-19 treatment
now fully approved for use by the FDA
✓ NOVEL
✔ RAPID
EFFECTIVE
FDA U.S. FOOD & DRUG
ADMINISTRATION
Our technology and Al workflows identified a previously
unknown antiviral mechanism (¹)
The Benevolent PlatformTM empowered scientists to
rapidly formulate a hypothesis in just 48 hours
Baricitinib shown to reduce mortality from COVID-19 in
randomised controlled trials: COV-BARRIER trial showed
baricitinib reduces mortality by 38% in hospitalised
patients(2), and by 46% in ventilated or ECMO patients (3)
FDA approved the use of baricitinib to treat COVID-19 in
May 2022 (4) after first granting emergency use authorisation
for baricitinib in combination with remdesivir in Nov 2020 (5)
Sources: (1) Richardson et al. Lancet 2020; (2) Marconi et al. Lancet Respiratory Medicine 2021; (3) Ely et al.
Lancet Respiratory Medicine 2022; (4) Lilly press release 11 May 2022; (5) Lilly press release 19 Nov 2020
BenevolentAl published
research in Feb 2020(¹)
THE LANCET
Led to equity investment
from Eli Lilly
Lilly
Benevolent 25View entire presentation