Ocuphire Pharma Results Presentation Deck
RM
24
Reversal of Mydriasis (RM) - Acute Treatment
Single Use Indication Leveraging a Precedent Approval Pathway
●
●
●
●
Nyxol's Potential Differentiated Solution
Regulatory Precedent with Rev-Eyes (an alpha 1
blocker), approved by the FDA in 1990 but shortly
thereafter discontinued (not for safety or efficacy
reasons)
Clinical Effect to potentially reduce pupil size and
counteract the effect of mydriatic drugs (alpha
agonists and cholinergic blockers) used to dilate
the pupil
Convenient eye drop given at the office that may
allow vision to return to normal sooner
Tolerable with a minimal side effect profile (unlike
cholinergic agonists such as pilocarpine)
Source: GlobalData Market Research Report, 2020
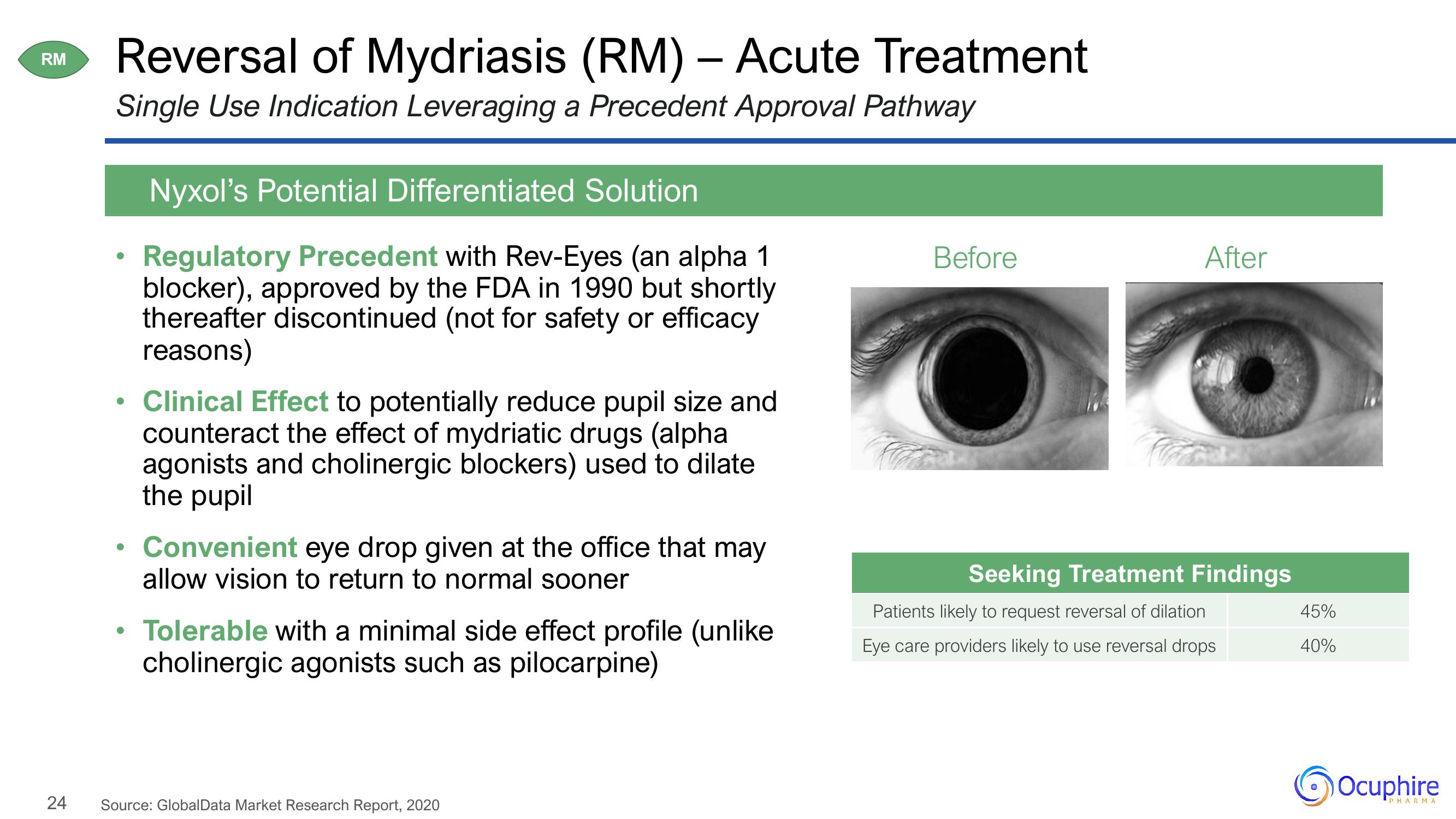
Before
After
Seeking Treatment Findings
Patients likely to request reversal of dilation
Eye care providers likely to use reversal drops
45%
40%
Ocuphire
PHARMAView entire presentation