AstraZeneca Results Presentation Deck
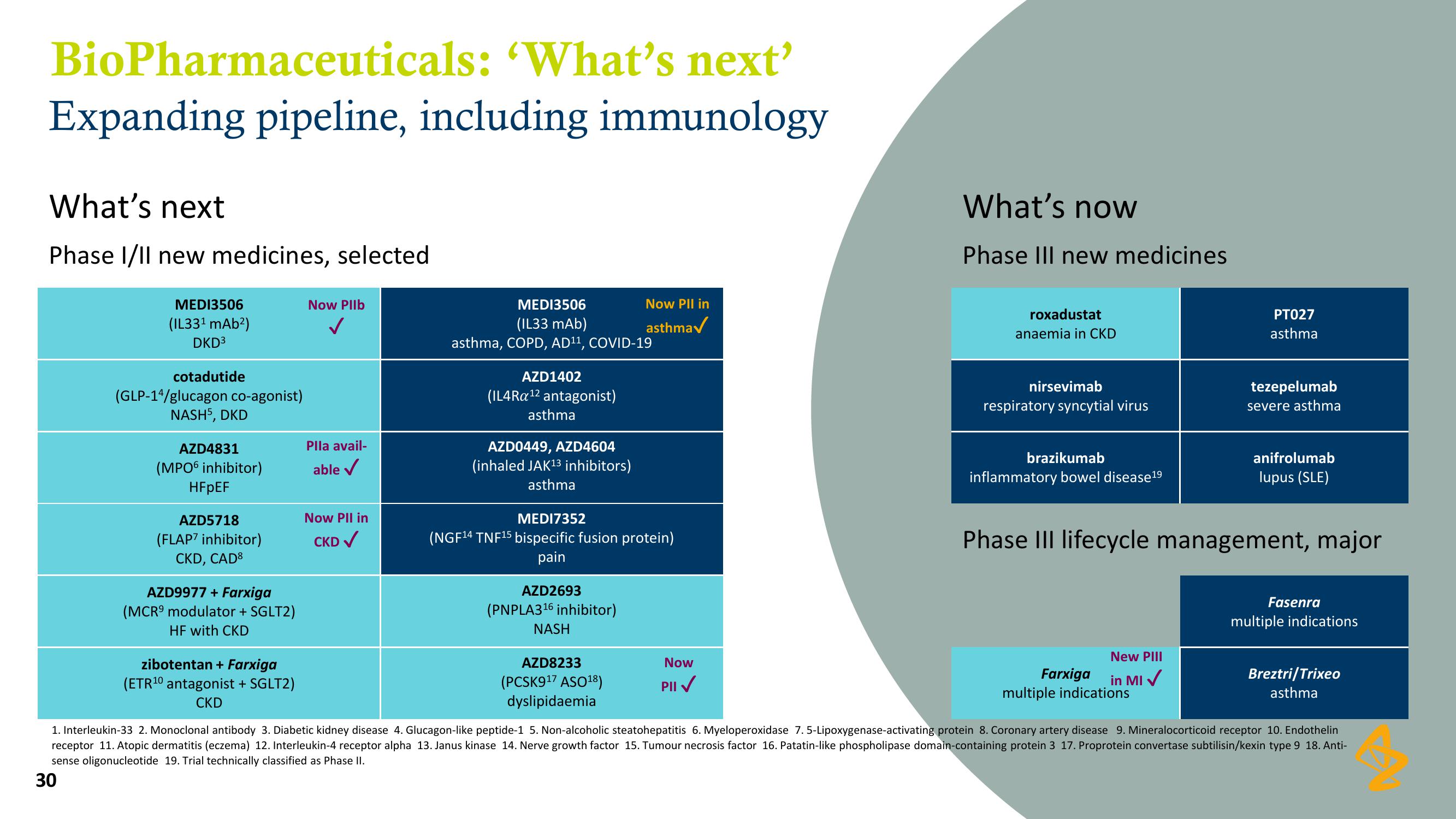
BioPharmaceuticals:
'What's next'
Expanding pipeline, including immunology
What's next
Phase I/II new medicines, selected
MEDI3506
(IL33¹ mAb²)
DKD³
cotadutide
(GLP-14/glucagon co-agonist)
NASH5, DKD
AZD4831
(MPO6 inhibitor)
HFpEF
AZD5718
(FLAP7 inhibitor)
CKD, CAD8
AZD9977 + Farxiga
(MCR⁹ modulator + SGLT2)
HF with CKD
zibotentan + Farxiga
(ETR¹0 antagonist + SGLT2)
CKD
Now Pllb
Plla avail-
able ✓
Now Pll in
CKD ✓
MEDI3506
(IL33 mAb)
asthma, COPD, AD¹¹, COVID-19
AZD1402
(IL4Ra ¹2 antagonist)
asthma
AZD0449, AZD4604
(inhaled JAK¹3 inhibitors)
asthma
MEDI7352
(NGF¹4 TNF¹5 bispecific fusion protein)
pain
AZD2693
(PNPLA3¹6 inhibitor)
NASH
Now Pll in
asthma
AZD8233
(PCSK9¹7 ASO¹8)
dyslipidaemia
Now
PII✓
What's now
Phase III new medicines
roxadustat
anaemia in CKD
nirsevimab
respiratory syncytial virus
brazikumab
inflammatory bowel disease19
PT027
asthma
New PIII
Farxiga
in MI✓
multiple indications
tezepelumab
severe asthma
anifrolumab
lupus (SLE)
Phase III lifecycle management, major
Fasenra
multiple indications
Breztri/Trixeo
asthma
1. Interleukin-33 2. Monoclonal antibody 3. Diabetic kidney disease 4. Glucagon-like peptide-1 5. Non-alcoholic steatohepatitis 6. Myeloperoxidase 7.5-Lipoxygenase-activating protein 8. Coronary artery disease 9. Mineralocorticoid receptor 10. Endothelin
receptor 11. Atopic dermatitis (eczema) 12. Interleukin-4 receptor alpha 13. Janus kinase 14. Nerve growth factor 15. Tumour necrosis factor 16. Patatin-like phospholipase domain-containing protein 3 17. Proprotein convertase subtilisin/kexin type 9 18. Anti-
sense oligonucleotide 19. Trial technically classified as Phase II.
30View entire presentation