BioNTech Investor Day Presentation Deck
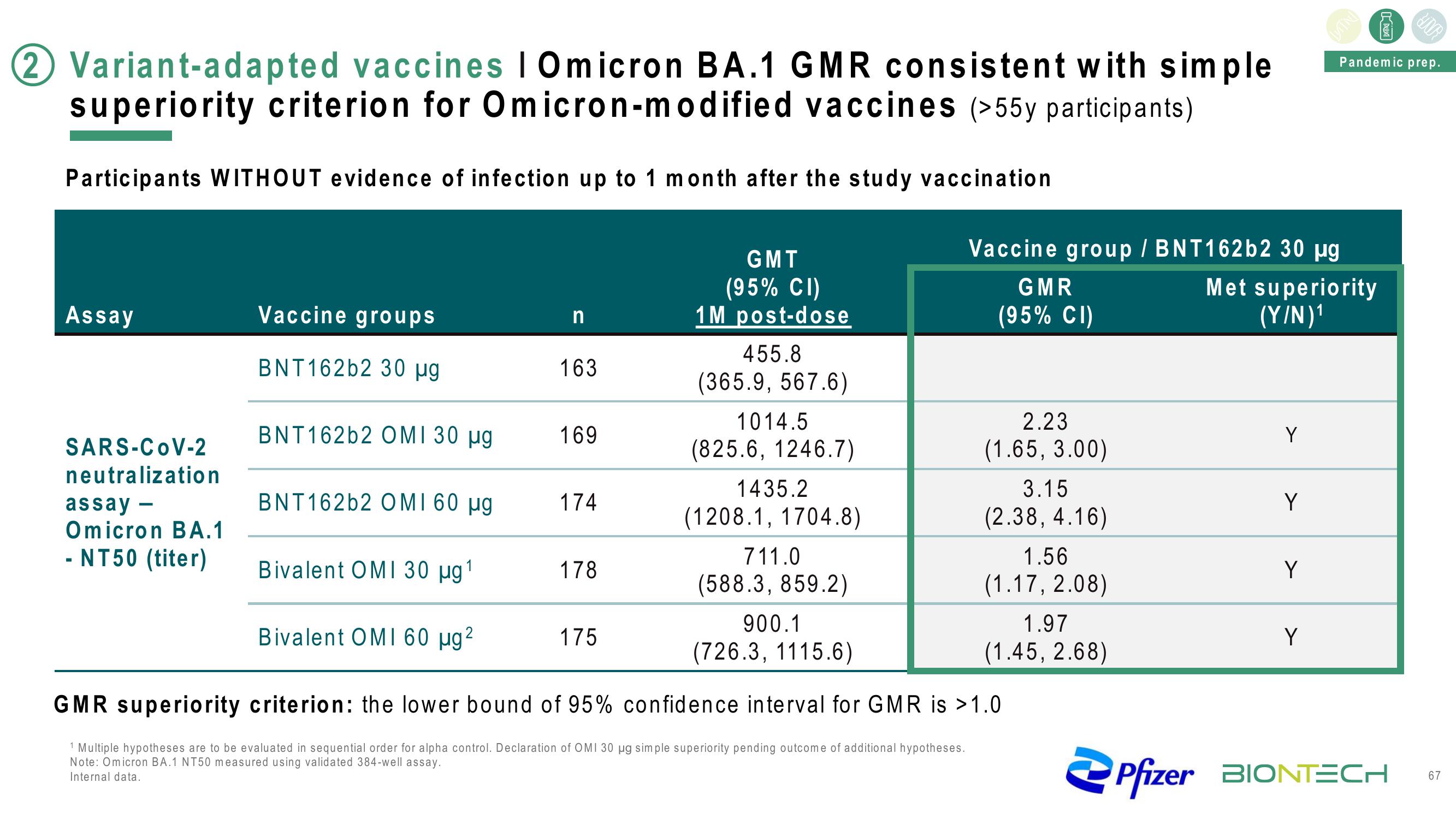
2 Variant-adapted vaccines | Omicron BA.1 GMR consistent with simple
superiority criterion for Omicron-modified vaccines (>55y participants)
Participants WITHOUT evidence of infection up to 1 month after the study vaccination
Assay
SARS-CoV-2
neutralization
assay -
Omicron BA.1
- NT50 (titer)
Vaccine groups
BNT162b2 30 µg
BNT162b2 OMI 30 μg
BNT162b2 OMI 60 μµg
n
163
169
174
178
GMT
(95% CI)
1M post-dose
455.8
(365.9, 567.6)
1014.5
(825.6, 1246.7)
1435.2
(1208.1, 1704.8)
175
711.0
(588.3, 859.2)
900.1
(726.3, 1115.6)
Vaccine group / BNT162b2 30 μg
GMR
(95% CI)
2.23
(1.65, 3.00)
3.15
(2.38, 4.16)
Bivalent OMI 30 μg ¹
Bivalent OMI 60 µg ²
GMR superiority criterion: the lower bound of 95% confidence interval for GMR is >1.0
1 Multiple hypotheses are to be evaluated in sequential order for alpha control. Declaration of OMI 30 µg simple superiority pending outcome of additional hypotheses.
Note: Omicron BA.1 NT50 measured using validated 384-well assay.
Internal data.
1.56
(1.17, 2.08)
1.97
(1.45, 2.68)
Met superiority
(Y/N)¹
Y
Y
Y
Y
NUA
Pandemic prep.
CAR
Pfizer BIONTECH
Pfizer
67View entire presentation