AstraZeneca Investor Conference Presentation Deck
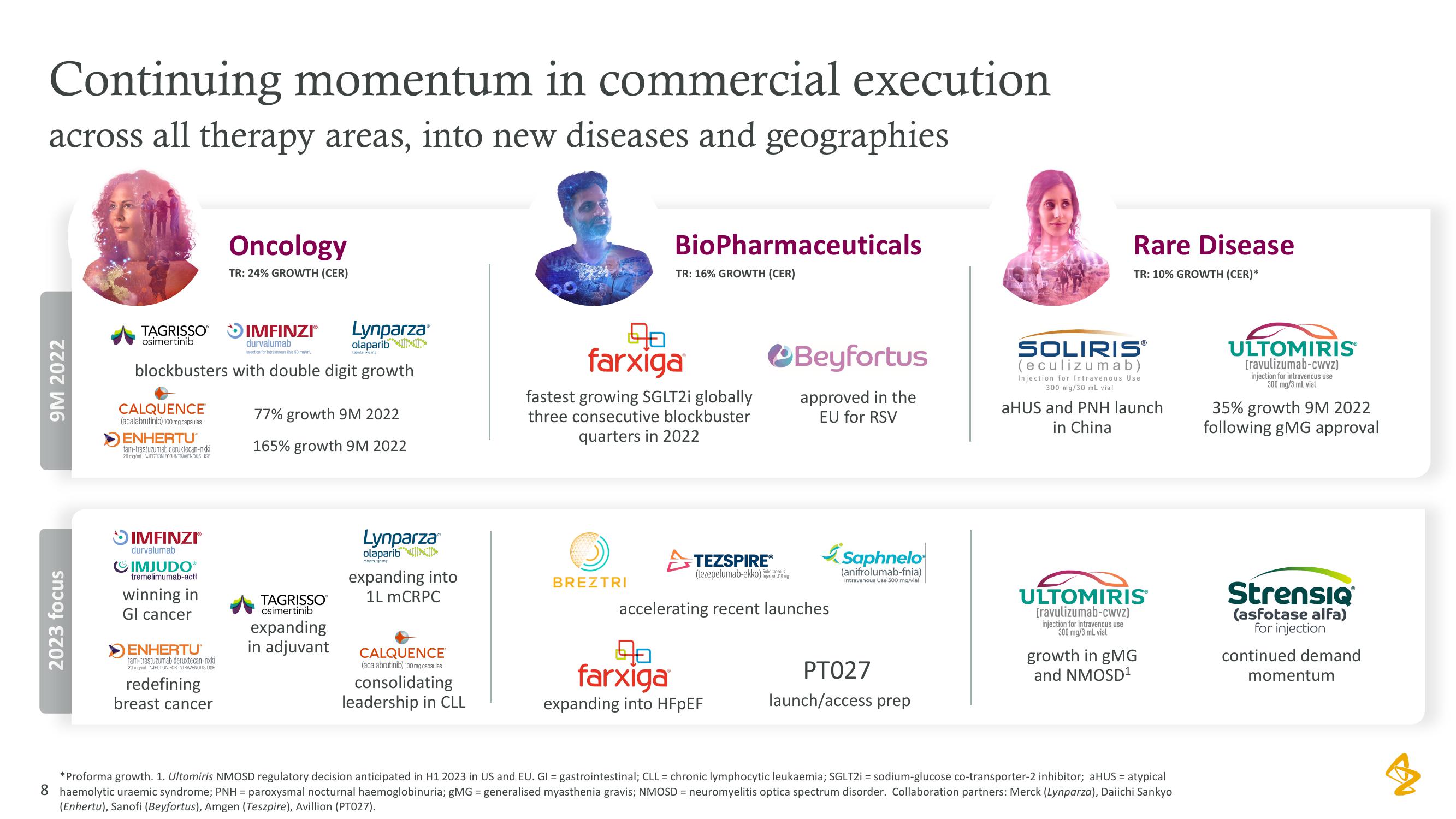
Continuing momentum in commercial execution
across all therapy areas, into new diseases and geographies
9M 2022
2023 focus
TAGRISSOⓇ IMFINZIⓇ
osimertinib
CALQUENCE
(acalabrutinib) 100 mg capsules
ENHERTUⓇ
fam-trastuzumab deruxtecan-nxki
20 mg/mL INJECTION FOR INTRAVENOUS USE
durvalumab
Injection for Intravenous Use 50 mg/ml
blockbusters with double digit growth
IMFINZIⓇ
durvalumab
CIMJUDOⓇ
tremelimumab-actl
winning in
Gl cancer
Oncology
TR: 24% GROWTH (CER)
DENHERTU'
tam-trastuzumab deruxtecan-nxki
20 mg/mL INJECTION FOR INTRAVENOUS USE
redefining
breast cancer
Lynparza
olaparib
77% growth 9M 2022
165% growth 9M 2022
TAGRISSO
osimertinib
expanding
in adjuvant
Lynparza
olaparib s
tablets 150 mg
expanding into
1L mCRPC
CALQUENCE
(acalabrutinib) 100 mg capsules
consolidating
leadership in CLL
BioPharmaceuticals
TR: 16% GROWTH (CER)
90
farxiga
fastest growing SGLT2i globally
three consecutive blockbuster
quarters in 2022
BREZTRI
TEZSPIREⓇ
(tezepelumab-ekko) injection 210 mg
accelerating recent launches
Beyfortus
approved in the
EU for RSV
9.0
farxiga
expanding into HFpEF
Saphnelo
(anifrolumab-fnia)
Intravenous Use 300 mg/vial
PT027
launch/access prep
Rare Disease
TR: 10% GROWTH (CER)*
SOLIRISⓇ
(eculizumab)
Injection for Intravenous Use
300 mg/30 mL vial
aHUS and PNH launch
in China
ULTOMIRIS®
(ravulizumab-cwvZ)
injection for intravenous use
300 mg/3 mL vial
growth in gMG
and NMOSD¹
*Proforma growth. 1. Ultomiris NMOSD regulatory decision anticipated in H1 2023 in US and EU. GI = gastrointestinal; CLL = chronic lymphocytic leukaemia; SGLT2i = sodium-glucose co-transporter-2 inhibitor; aHUS = atypical
8 haemolytic uraemic syndrome; PNH = paroxysmal nocturnal haemoglobinuria; gMG = generalised myasthenia gravis; NMOSD = neuromyelitis optica spectrum disorder. Collaboration partners: Merck (Lynparza), Daiichi Sankyo
(Enhertu), Sanofi (Beyfortus), Amgen (Teszpire), Avillion (PT027).
ULTOMIRISⓇ
(ravulizumab-cwvVZ)
injection for intravenous use
300 mg/3 mL vial
35% growth 9M 2022
following gMG approval
Strensio
(asfotase alfa)
for injection
continued demand
momentum
3View entire presentation