Imara M&A
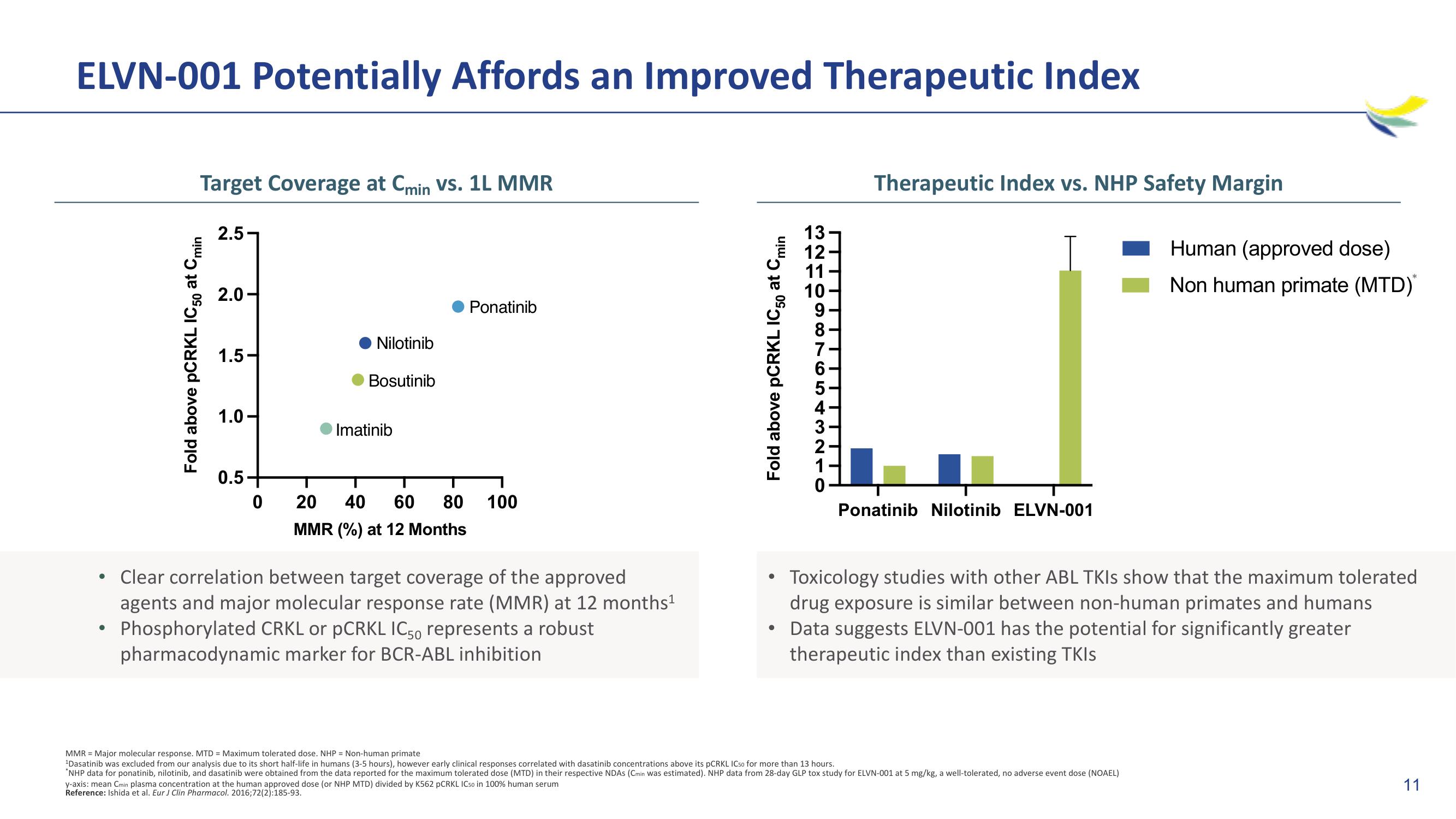
ELVN-001 Potentially Affords an Improved Therapeutic Index
●
●
Target Coverage at Cmin vs. 1L MMR
Fold above PCRKL IC50 at Cmin
2.5
2.0-
1.5-
1.0-
0.5-
0
Nilotinib
Bosutinib
Imatinib
Ponatinib
20 40 60 80 100
MMR (%) at 12 Months
Clear correlation between target coverage of the approved
agents and major molecular response rate (MMR) at 12 months¹
Phosphorylated CRKL or PCRKL IC50 represents a robust
pharmacodynamic marker for BCR-ABL inhibition
Fold above pCRKL IC50 at Cmin
13-
12
11
10
●
32109 BTCF43210
Therapeutic Index vs. NHP Safety Margin
Ponatinib Nilotinib ELVN-001
Toxicology studies with other ABL TKIs show that the maximum tolerated
drug exposure is similar between non-human primates and humans
• Data suggests ELVN-001 has the potential for significantly greater
therapeutic index than existing TKIs
Human (approved dose)
Non human primate (MTD)
MMR = Major molecular response. MTD = Maximum tolerated dose. NHP = Non-human primate
¹Dasatinib was excluded from our analysis due to its short half-life in humans (3-5 hours), however early clinical responses correlated with dasatinib concentrations above its pCRKL IC50 for more than 13 hours.
*NHP data for ponatinib, nilotinib, and dasatinib were obtained from the data reported for the maximum tolerated dose (MTD) in their respective NDAS (Cmin was estimated). NHP data from 28-day GLP tox study for ELVN-001 at 5 mg/kg, a well-tolerated, no adverse event dose (NOAEL)
y-axis: mean Cmin plasma concentration at the human approved dose (or NHP MTD) divided by K562 pCRKL ICso in 100% human serum
Reference: Ishida et al. Eur J Clin Pharmacol. 2016;72(2):185-93.
11View entire presentation