Ocuphire Pharma Investor Day Presentation Deck
P
98
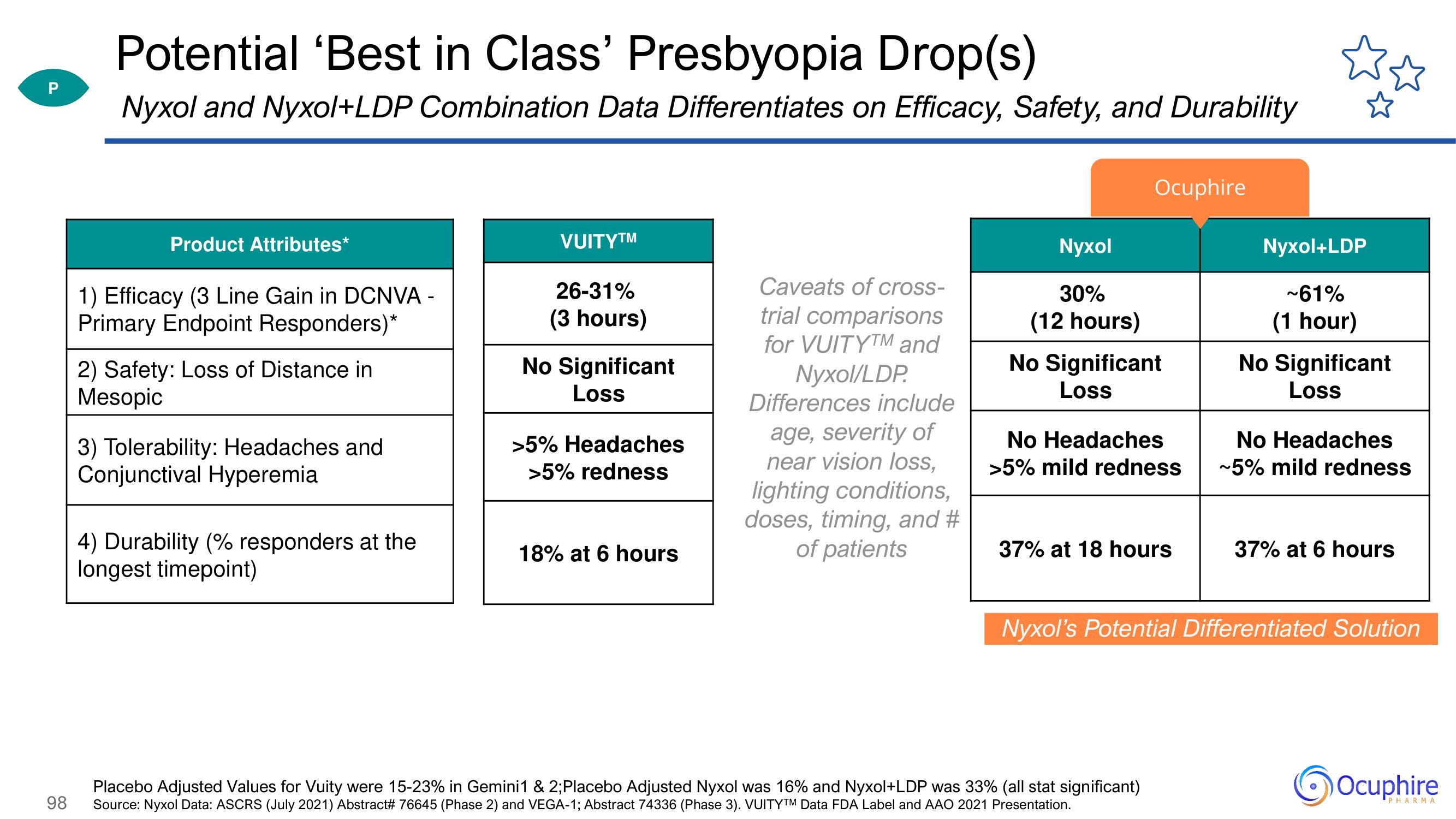
Potential 'Best in Class' Presbyopia Drop(s)
Nyxol and Nyxol+LDP Combination Data Differentiates on Efficacy, Safety, and Durability
Product Attributes*
1) Efficacy (3 Line Gain in DCNVA -
Primary Endpoint Responders)*
2) Safety: Loss of Distance in
Mesopic
3) Tolerability: Headaches and
Conjunctival Hyperemia
4) Durability (% responders at the
longest timepoint)
VUITY™
26-31%
(3 hours)
No Significant
Loss
>5% Headaches
>5% redness
18% at 6 hours
Caveats of cross-
trial comparisons
for VUITYTM and
Nyxol/LDP.
Differences include
age, severity of
near vision loss,
lighting conditions,
doses, timing, and #
of patients
Nyxol
30%
(12 hours)
Ocuphire
No Significant
Loss
No Headaches
>5% mild redness
37% at 18 hours
Placebo Adjusted Values for Vuity were 15-23% in Gemini1 & 2; Placebo Adjusted Nyxol was 16% and Nyxol+LDP was 33% (all stat significant)
Source: Nyxol Data: ASCRS (July 2021) Abstract# 76645 (Phase 2) and VEGA-1; Abstract 74336 (Phase 3). VUITYTM Data FDA Label and AAO 2021 Presentation.
Nyxol+LDP
~61%
(1 hour)
No Significant
Loss
No Headaches
~5% mild redness
37% at 6 hours
Nyxol's Potential Differentiated Solution
Ocuphire
PHARMAView entire presentation