Vaxcyte Corporate Presentation
Critical Manufacturing Foundation Established for PCV Franchise
Designed to Provide Robust & Scalable Capacity to Independently Supply Market
27
27
Strategic Alignment with Best-in-Class CDMO
Lonza +
Overview / Structure:
•
•
End-to-end "turnkey" supply established at marquee Swiss facility
Fee-for-service relationship with risk sharing to align the parties
Status:
•
•
·
•
•
•
•
Manufactured, tested and released GMP critical raw materials (eCRMⓇ & 24
polysaccharides)
Manufactured, tested and released the 24 GMP conjugated drug substances (DS)
Completed GMP drug product (DP) manufacture (formulation, fill and finish)
Anticipated completion of remaining DP testing and release, as well as
documentation of stability, is expected prior to IND application filing and supply for
VAX-24 Phase 1/2 clinical development
Commercial production capacity available at same site using existing infrastructure
or Ibex capacity coming on-line
Exclusive License to
Cell-Free Protein Synthesis Platform
SUTRO
BIOPHARMA
Exclusive, worldwide, royalty-bearing, sub-licensable license for field of
vaccines to treat or prevent infectious disease (4% royalty)
Sutro Biopharma source of cell-free extract and custom reagents
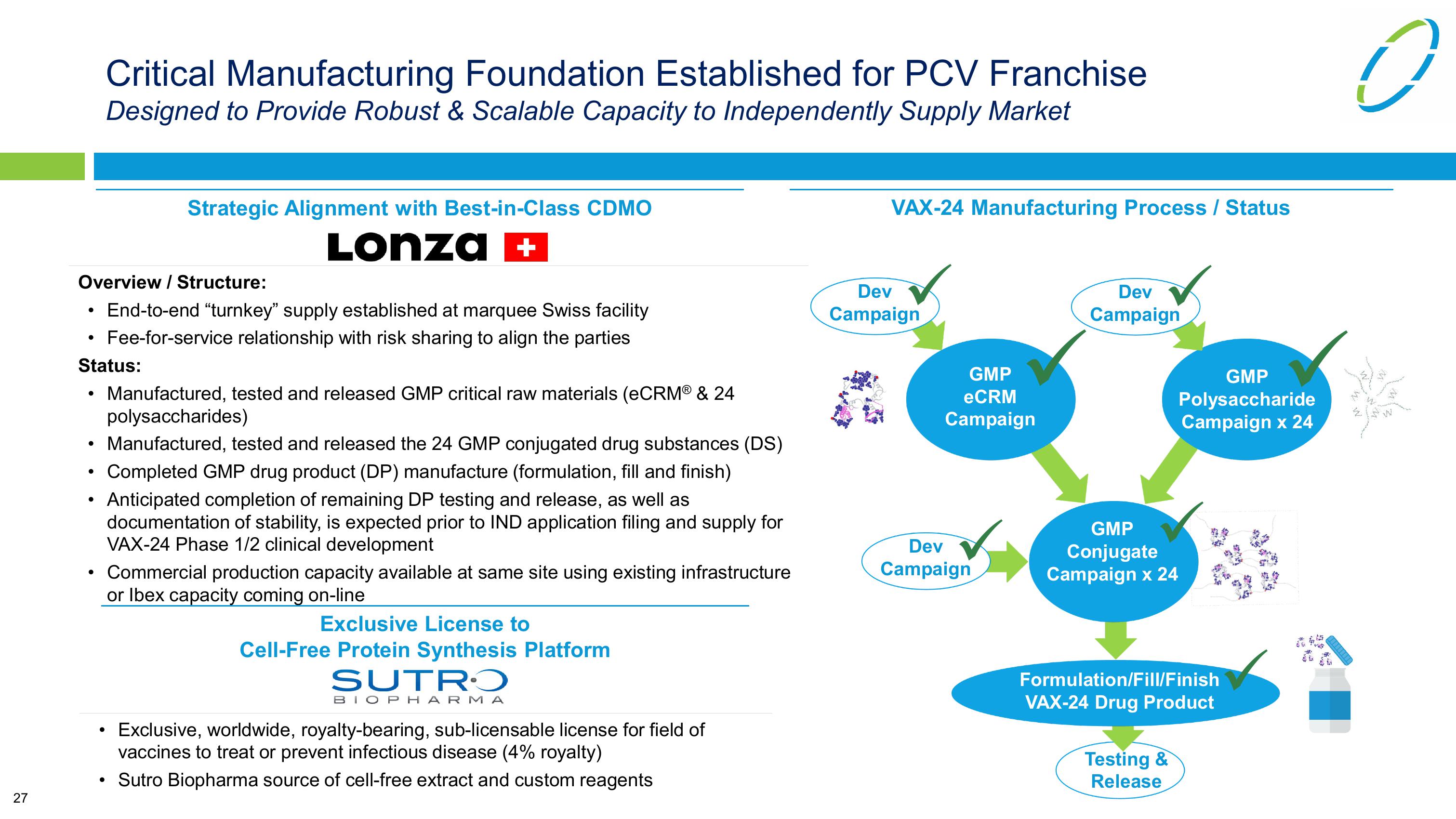
VAX-24 Manufacturing Process / Status
Dev
Campaign
GMP
eCRM
Campaign
Dev
Campaign
Dev
Campaign
GMP
Conjugate
Campaign x 24
GMP
Polysaccharide
Campaign x 24
Formulation/Fill/Finish
VAX-24 Drug Product
Testing &
Release
оView entire presentation