Coppersmith Presentation to Alere Inc Stockholders
PAGE 18 |
FDA Issues: Poor Execution, Lack of Preparation & Self-Serving Reporting
▪ Intrinsic operational problems were compounded by FDA issues relating to Alere's Triage troponin product line
> March 2012, FDA began investigation into the alignment between the labeling and quality control release specifications
> May 2012, Alere announced product recalls and changes in release specifications which lowered manufacturing yields
> 14.7% decline in stock price on the announcement of the recall
> Negatively impacted 2012 revenue by $49mm and EPS by $0.29-$0.32, we estimate¹1
>
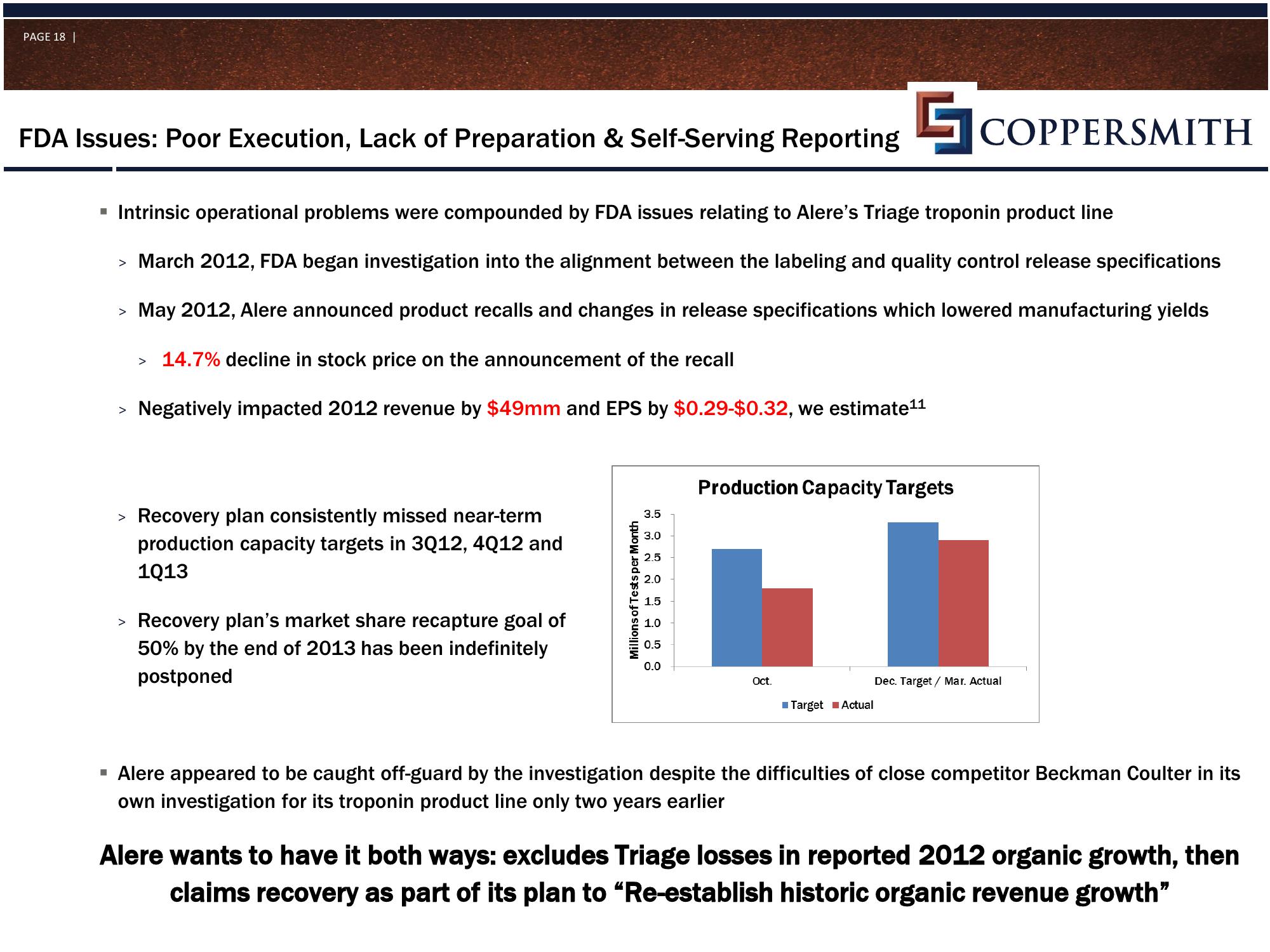
Recovery plan consistently missed near-term
production capacity targets in 3Q12, 4Q12 and
1Q13
> Recovery plan's market share recapture goal of
50% by the end of 2013 has been indefinitely
postponed
Millions of Tests per Month
3.5
3.0
2.5
2.0
1.5
1.0
0.5
0.0
Go COPPERSMITH
Production Capacity Targets
Oct.
Target Actual
Dec. Target / Mar. Actual
▪ Alere appeared to be caught off-guard by the investigation despite the difficulties of close competitor Beckman Coulter in its
own investigation for its troponin product line only two years earlier
Alere wants to have it both ways: excludes Triage losses in reported 2012 organic growth, then
claims recovery as part of its plan to "Re-establish historic organic revenue growth"View entire presentation