23andMe Investor Day Presentation Deck
PFS probability (%)
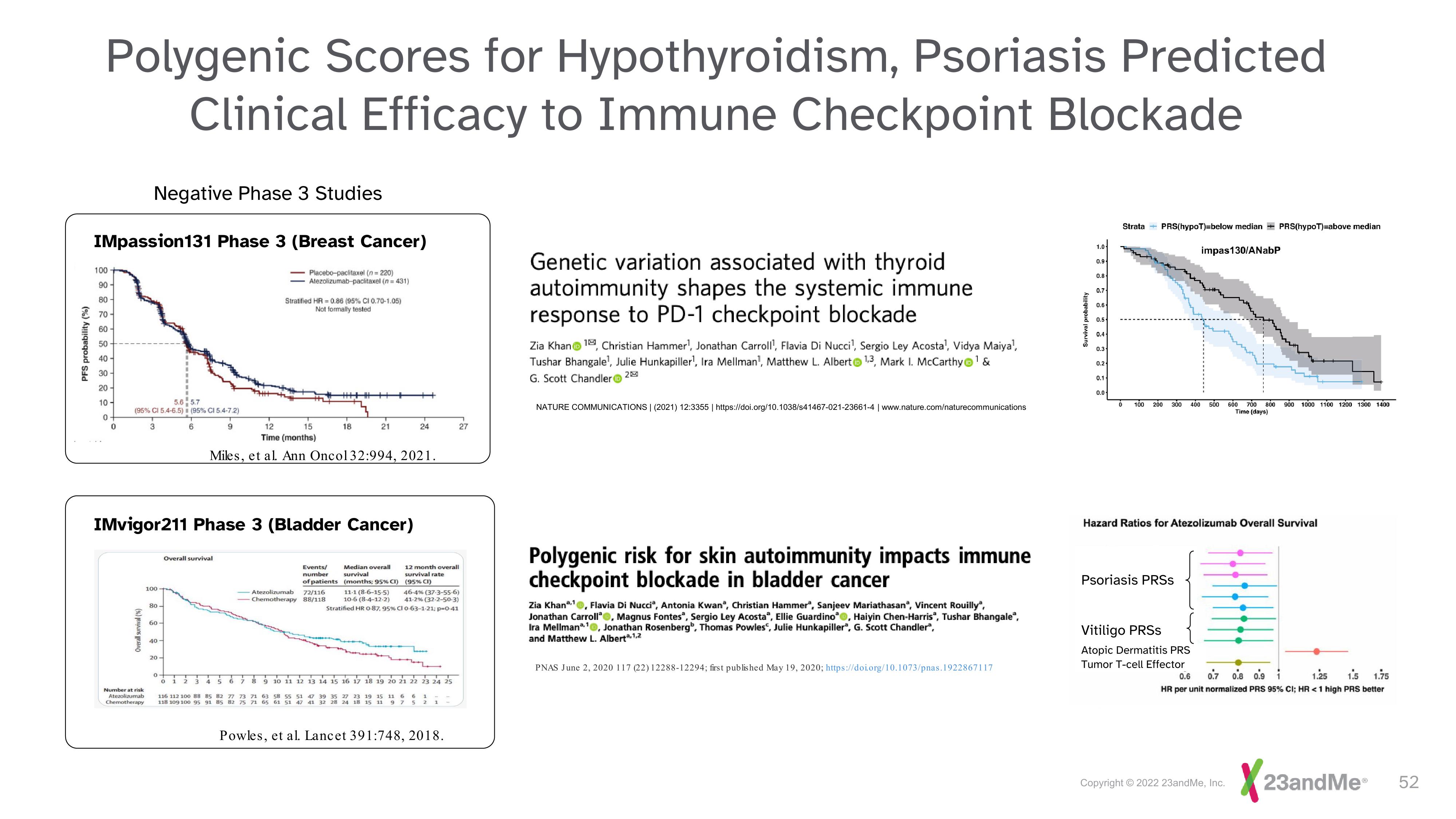
Polygenic Scores for Hypothyroidism, Psoriasis Predicted
Clinical Efficacy to Immune Checkpoint Blockade
IMpassion131 Phase 3 (Breast Cancer)
Placebo-paclitaxel (n=220)
Atezolizumab-paclitaxel (n=431)
100
90
80-
70-
60
50
40
30
20
10-
0
Negative Phase 3 Studies
5.6 5.7
(95% CI 5.4-6.5) (95% CI 5.4-7.2)
6
9
Overall survival (%)
Number at risk
Atezolizumab
Chemotherapy
100
IMvigor211 Phase 3 (Bladder Cancer)
80-
60-
40-
20-
Stratified HR-0.86 (95% CI 0.70-1.05)
Not formally tested
12
15
Time (months)
Miles, et al. Ann Oncol 32:994, 2021.
Overall survival
18
21
Events/
number
of patients
-Atezolizumab 72/116
Chemotherapy 88/118
24
12 month overall
survival rate
(95% CI)
Median overall
survival
(months; 95% CI)
11-1 (8-6-15-5) 46-4% (37-3-55-6)
10-6 (8-4-12-2) 41-2% (32-2-50-3)
Stratified HR 0-87, 95% CI 0-63-1-21: p-0-41
Leave
0
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
112 100 88 85 82 77 73 58 55 47
118 109 100 95 91 85 82 75 71 65 61 51 47 41 32 28 24 18 15 11 92821
27
Powles, et al. Lancet 391:748, 2018.
Genetic variation associated with thyroid
autoimmunity shapes the systemic immune
response to PD-1 checkpoint blockade
Zia Khan ¹, Christian Hammer¹, Jonathan Carroll, Flavia Di Nucci¹, Sergio Ley Acosta¹, Vidya Maiya',
Tushar Bhangale¹, Julie Hunkapiller¹, Ira Mellman¹, Matthew L. Albert 1,3, Mark I. McCarthy ¹ &
G. Scott Chandler 2
NATURE COMMUNICATIONS | (2021) 12:3355 | https://doi.org/10.1038/s41467-021-23661-4 | www.nature.com/naturecommunications
Polygenic risk for skin autoimmunity impacts immune
checkpoint blockade in bladder cancer
Zia Khan,¹, Flavia Di Nucci", Antonia Kwan", Christian Hammer", Sanjeev Mariathasan", Vincent Rouilly",
Jonathan Carroll, Magnus Fontes, Sergio Ley Acosta, Ellie Guardino, Haiyin Chen-Harris, Tushar Bhangale",
Ira Mellman ³¹, Jonathan Rosenberg, Thomas Powles, Julie Hunkapiller", G. Scott Chandler",
and Matthew L. Albert1.2
PNAS June 2, 2020 117 (22) 12288-12294; first published May 19, 2020; https://doi.org/10.1073/pnas.1922867117
Survival probability
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0.0
Strata
PRS(hypoT)-below median+ PRS(hypoT)-above median
0 100 200 300 400 500 600 700 800 900 1000 1100 1200 1300 1400
Time (days)
impas130/ANabP
Hazard Ratios for Atezolizumab Overall Survival
Psoriasis PRSS
Vitiligo PRSs
Atopic Dermatitis PRS
Tumor T-cell Effector
•
●
0.6 0.7 0.8 0.9 1 1.25 1.5 1.75
HR per unit normalized PRS 95% CI; HR <1 high PRS better
Copyright © 2022 23andMe, Inc.
23andMe®
52View entire presentation