Kymera Investor Presentation Deck
●
Partial Response and Stable Disease Observed Across Multiple Tumor
Types During Dose Escalation of KT-333
ASH Poster Presentation (Data Cut-off of October 18, 2023)
●
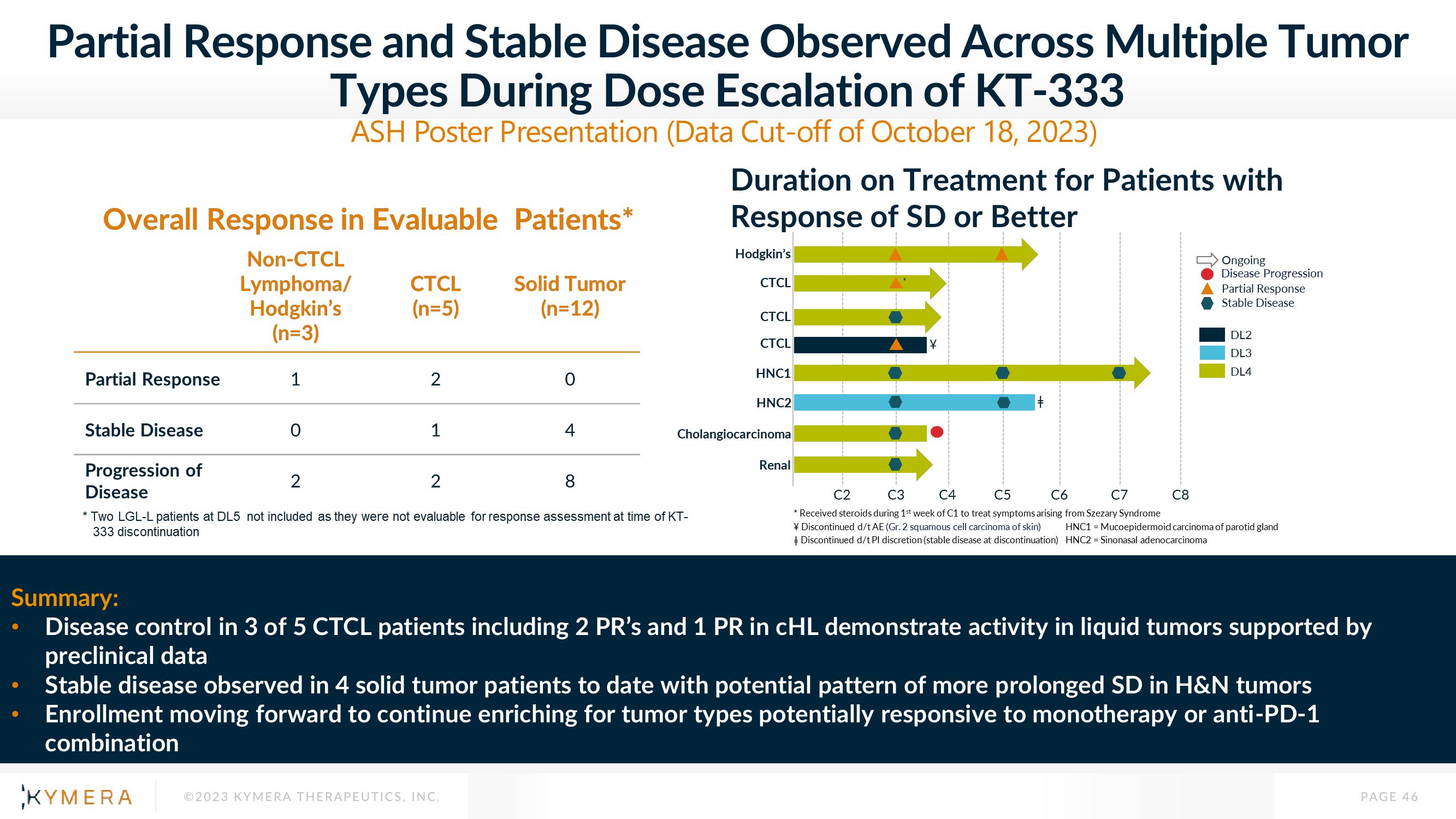
Overall Response in Evaluable Patients*
Non-CTCL
Lymphoma/
Hodgkin's
(n=3)
Partial Response
Stable Disease
Progression of
Disease
1
0
CTCL
(n=5)
2
1
Solid Tumor
(n=12)
0
4
KYMERA ©2023 KYMERA THERAPEUTICS, INC.
2
2
8
* Two LGL-L patients at DL5 not included as they were not evaluable for response assessment at time of KT-
333 discontinuation
Duration on Treatment for Patients with
Response of SD or Better
Hodgkin's
CTCL
CTCL
CTCL
HNC1
HNC2
Cholangiocarcinoma
Renal
¥
Ongoing
Disease Progression
Partial Response
Stable Disease
C8
Summary:
Disease control in 3 of 5 CTCL patients including 2 PR's and 1 PR in cHL demonstrate activity in liquid tumors supported by
preclinical data
DL2
DL3
DL4
C2
C3
C4
C5
C6
C7
* Received steroids during 1st week of C1 to treat symptoms arising from Szezary Syndrome
¥ Discontinued d/t AE (Gr. 2 squamous cell carcinoma of skin) HNC1 = Mucoepidermoid carcinoma of parotid gland
Discontinued d/t Pl discretion (stable disease at discontinuation) HNC2 = Sinonasal adenocarcinoma
Stable disease observed in 4 solid tumor patients to date with potential pattern of more prolonged SD in H&N tumors
Enrollment moving forward to continue enriching for tumor types potentially responsive to monotherapy or anti-PD-1
combination
PAGE 46View entire presentation