Kymera Investor Presentation Deck
Mean (± SE) Percent IRAK4
Change from Baseline
50
-20
-40
-60
-80
-100
●
●
●
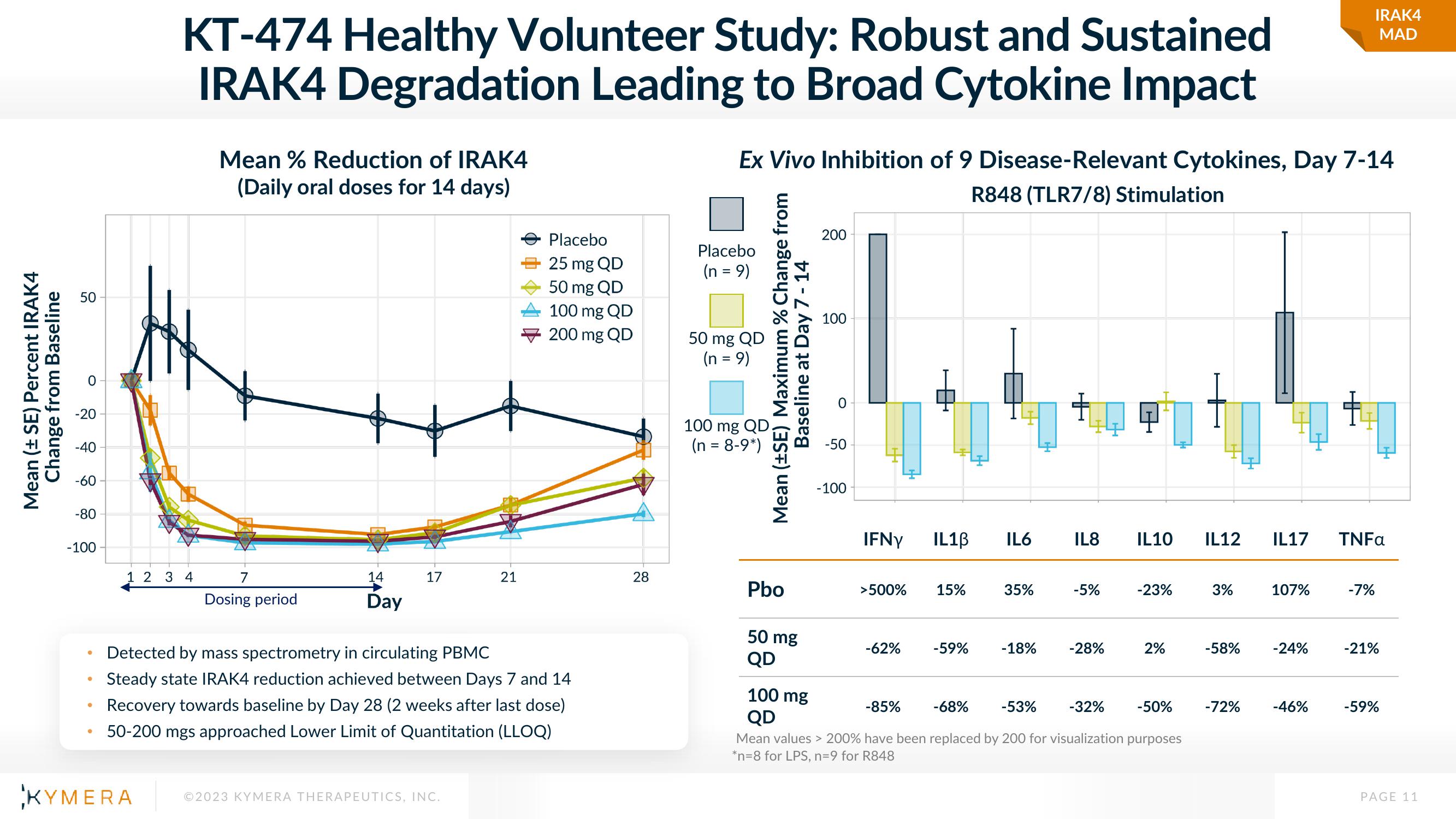
KT-474 Healthy Volunteer Study: Robust and Sustained
IRAK4 Degradation Leading to Broad Cytokine Impact
1 2 3 4
KYMERA
Mean % Reduction of IRAK4
(Daily oral doses for 14 days)
7
Dosing period
14
Day
17
21
Detected by mass spectrometry in circulating PBMC
Steady state IRAK4 reduction achieved between Days 7 and 14
Recovery towards baseline by Day 28 (2 weeks after last dose)
50-200 mgs approached Lower Limit of Quantitation (LLOQ)
©2023 KYMERA THERAPEUTICS, INC.
Placebo
25 mg QD
50 mg QD
100 mg QD
200 mg QD
28
Ex Vivo Inhibition of 9 Disease-Relevant Cytokines, Day 7-14
R848 (TLR7/8) Stimulation
Placebo
(n = 9)
50 mg QD
(n = 9)
Mean (+SE) Maximum % Change from
Baseline at Day 7 - 14
100 mg QD.
(n = 8-9*)
Pbo
50 mg
QD
200
100
-50
-100
IFNY
>500%
-62%
IL18 IL6
15%
-59%
35%
-18%
IL8 IL10
-5%
-28%
-23%
2%
100 mg
-85%
-68%
-53% -32% -50%
QD
Mean values > 200% have been replaced by 200 for visualization purposes
*n=8 for LPS, n=9 for R848
IL12
3%
-58%
-72%
IL17
107%
-24%
-46%
IRAK4
MAD
TNFa
-7%
-21%
-59%
PAGE 11View entire presentation