AstraZeneca Results Presentation Deck
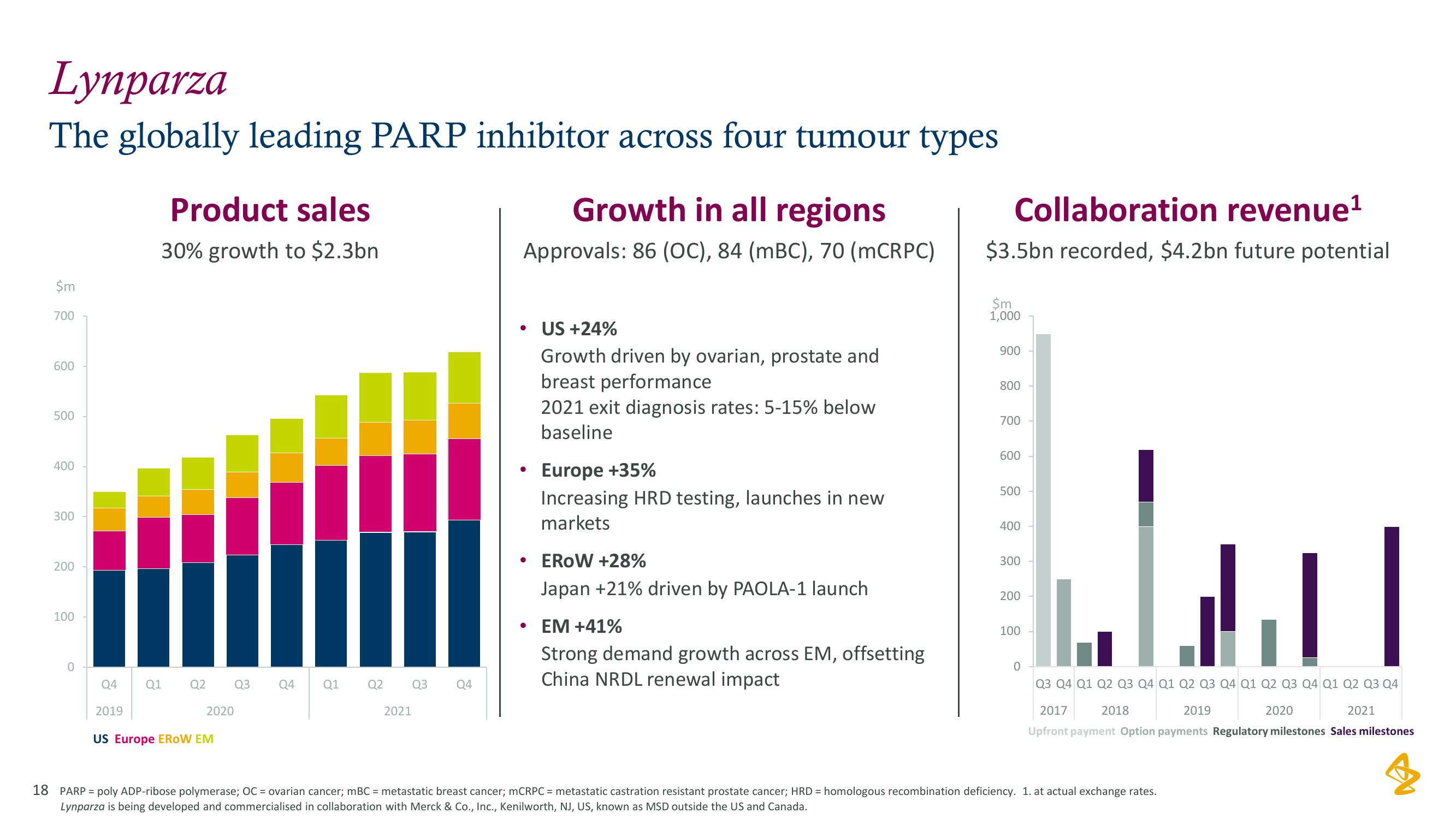
Lynparza
The globally leading PARP inhibitor across four tumour types
$m
700
600
500
400
300
200
100
0
Q4
2019
Product sales
30% growth to $2.3bn
Q1
Q2
2020
US Europe EROW EM
Q3
Q4
Q1
Q2
2021
Q3
Q4
Growth in all regions
Approvals: 86 (OC), 84 (mBC), 70 (mCRPC)
●
●
●
●
US +24%
Growth driven by ovarian, prostate and
breast performance
2021 exit diagnosis rates: 5-15% below
baseline
Europe +35%
Increasing HRD testing, launches in new
markets
EROW +28%
Japan +21% driven by PAOLA-1 launch
EM +41%
Strong demand growth across EM, offsetting
China NRDL renewal impact
Collaboration revenue¹
$3.5bn recorded, $4.2bn future potential
$m
1,000
900
800
700
600
500
400
300
200
100
0
Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4
2018
2017
2019
Upfront payment Option payments Regulatory milestones Sales milestones
||
18 PARP = poly ADP-ribose polymerase; OC = ovarian cancer; mBC = metastatic breast cancer; mCRPC = metastatic castration resistant prostate cancer; HRD = homologous recombination deficiency. 1. at actual exchange rates.
Lynparza is being developed and commercialised in collaboration with Merck & Co., Inc., Kenilworth, NJ, US, known as MSD outside the US and Canada.
2020
2021View entire presentation