AstraZeneca Results Presentation Deck
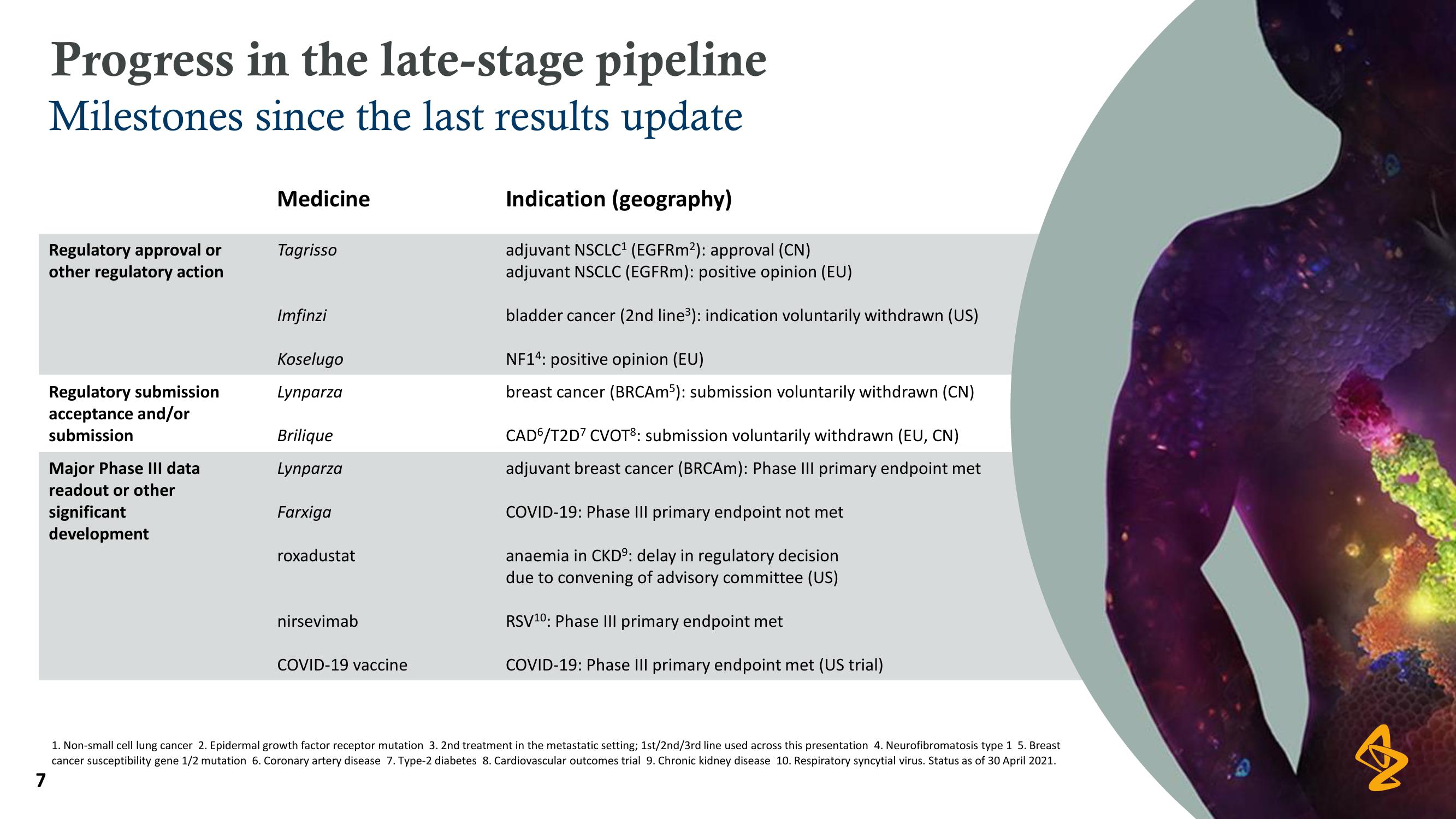
Progress in the late-stage pipeline
Milestones since the last results update
Regulatory approval or
other regulatory action
Regulatory submission
acceptance and/or
submission
Major Phase III data
readout or other
significant
development
Medicine
Tagrisso
Imfinzi
Koselugo
Lynparza
Brilique
Lynparza
Farxiga
roxadustat
nirsevimab
COVID-19 vaccine
Indication (geography)
adjuvant NSCLC¹ (EGFRm2): approval (CN)
adjuvant NSCLC (EGFRm): positive opinion (EU)
bladder cancer (2nd line³): indication voluntarily withdrawn (US)
NF14: positive opinion (EU)
breast cancer (BRCAm5): submission voluntarily withdrawn (CN)
CAD/T2D7 CVOT8: submission voluntarily withdrawn (EU, CN)
adjuvant breast cancer (BRCAm): Phase III primary endpoint met
COVID-19: Phase III primary endpoint not met
anaemia in CKDº: delay in regulatory decision
due to convening of advisory committee (US)
RSV10: Phase III primary endpoint met
COVID-19: Phase III primary endpoint met (US trial)
1. Non-small cell lung cancer 2. Epidermal growth factor receptor mutation 3. 2nd treatment in the metastatic setting; 1st/2nd/3rd line used across this presentation 4. Neurofibromatosis type 1 5. Breast
cancer susceptibility gene 1/2 mutation 6. Coronary artery disease 7. Type-2 diabetes 8. Cardiovascular outcomes trial 9. Chronic kidney disease 10. Respiratory syncytial virus. Status as of 30 April 2021.
7
4View entire presentation