Kymera Investor Day Presentation Deck
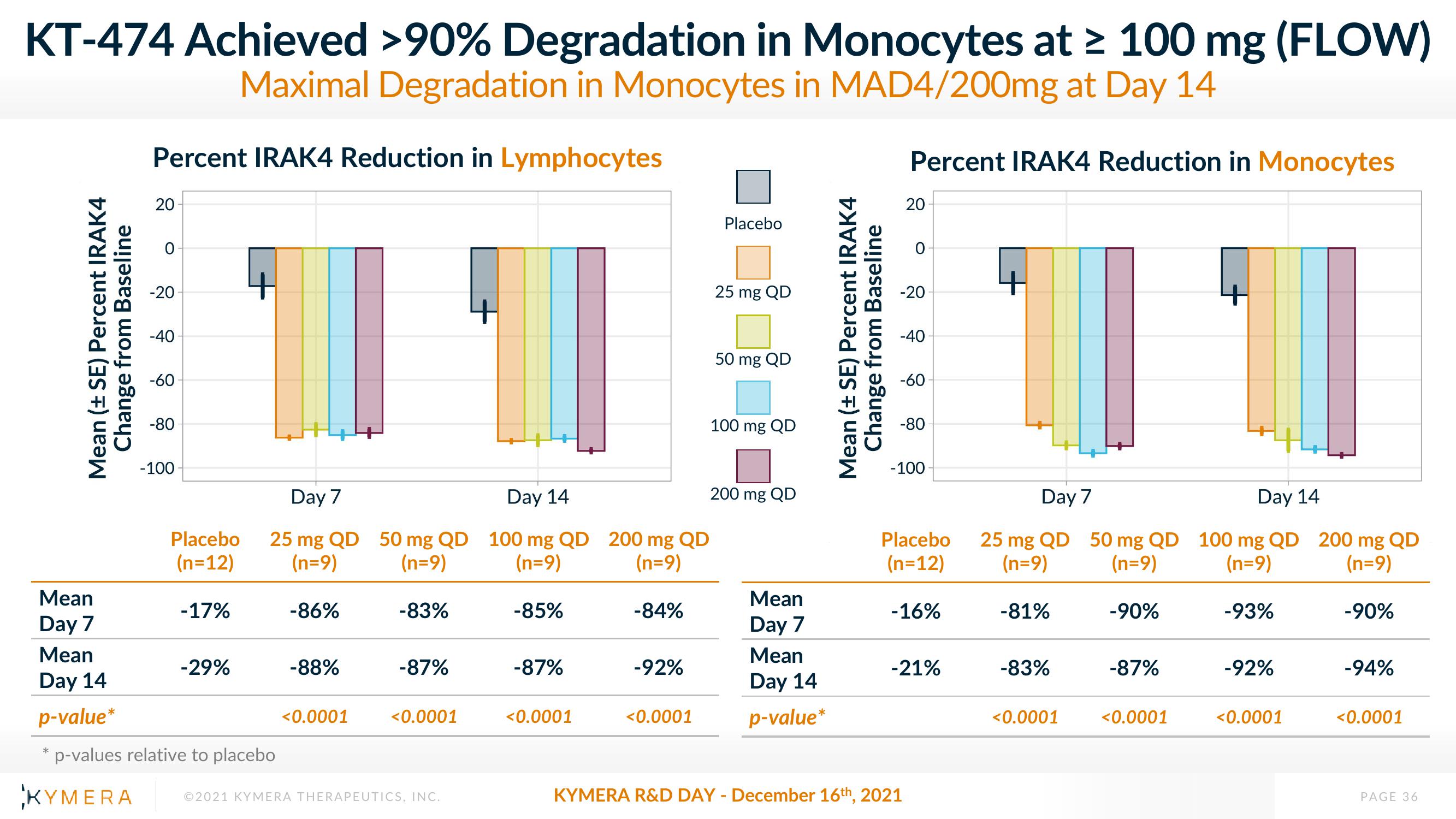
KT-474 Achieved >90% Degradation in Monocytes at ≥ 100 mg (FLOW)
Maximal Degradation in Monocytes in MAD4/200mg at Day 14
Percent IRAK4 Reduction in Lymphocytes
Percent IRAK4 Reduction in Monocytes
Mean (± SE) Percent IRAK4
Change from Baseline
Mean
Day 7
*
20
O
-20
-40
-60
-80
-100
Placebo
(n=12)
-17%
Day 7
25 mg QD 50 mg QD
(n=9) (n=9)
-83%
Mean
Day 14
p-value*
p-values relative to placebo
KYMERA ©2021 KYMERA THERAPEUTICS, INC.
-29%
-86%
-88%
<0.0001
-87%
<0.0001
Day 14
100 mg QD 200 mg QD
(n=9)
(n=9)
-85%
-87%
<0.0001
-84%
-92%
<0.0001
Placebo
25 mg QD
50 mg QD
100 mg QD
200 mg QD
Mean
Day 7
Mean
Day 14
p-value*
Mean (± SE) Percent IRAK4
Change from Baseline
20
O
-20
-40-
-60-
-80
-100-
Placebo
(n=12)
-16%
KYMERA R&D DAY - December 16th, 2021
-21%
Day 7
25 mg QD
(n=9)
-81%
-83%
<0.0001
50 mg QD
(n=9)
-90%
-87%
<0.0001
Day 14
100 mg QD 200 mg QD
(n=9)
(n=9)
-93%
-92%
<0.0001
-90%
-94%
<0.0001
PAGE 36View entire presentation