Ocuphire Pharma Investor Update
RM
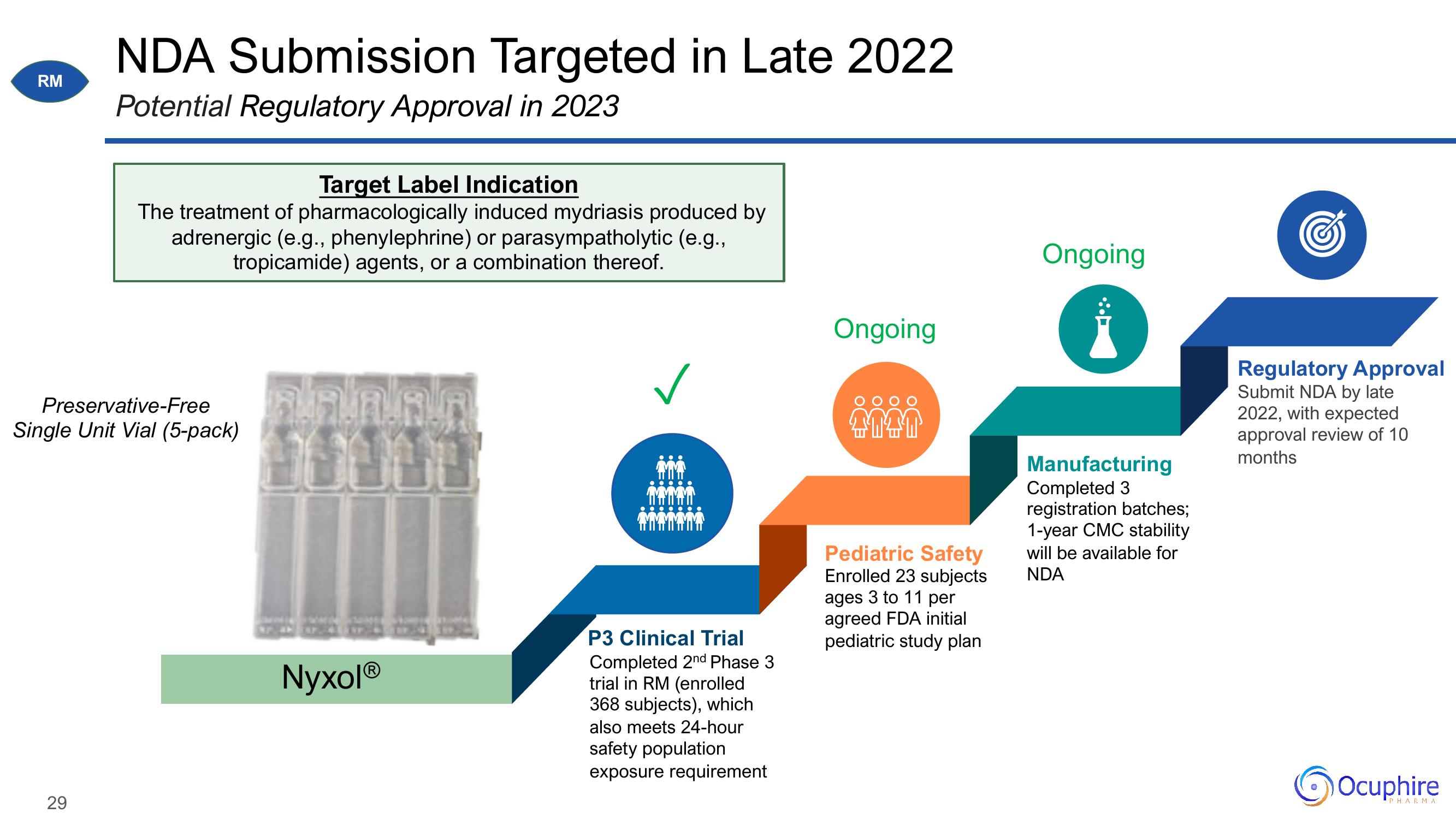
NDA Submission Targeted in Late 2022
Potential Regulatory Approval in 2023
29
Target Label Indication
The treatment of pharmacologically induced mydriasis produced by
adrenergic (e.g., phenylephrine) or parasympatholytic (e.g.,
tropicamide) agents, or a combination thereof.
Preservative-Free
Single Unit Vial (5-pack)
AAAAA
3000
NyxolⓇ
P3 Clinical Trial
Completed 2nd Phase 3
trial in RM (enrolled
368 subjects), which
also meets 24-hour
safety population
exposure requirement
Ongoing
4141
Pediatric Safety
Enrolled 23 subjects
ages 3 to 11 per
agreed FDA initial
pediatric study plan
Ongoing
Manufacturing
Completed 3
registration batches;
1-year CMC stability
will be available for
NDA
Regulatory Approval
Submit NDA by late
2022, with expected
approval review of 10
months
Ocuphire
PHARMAView entire presentation