Genelux Investor Presentation Deck
●
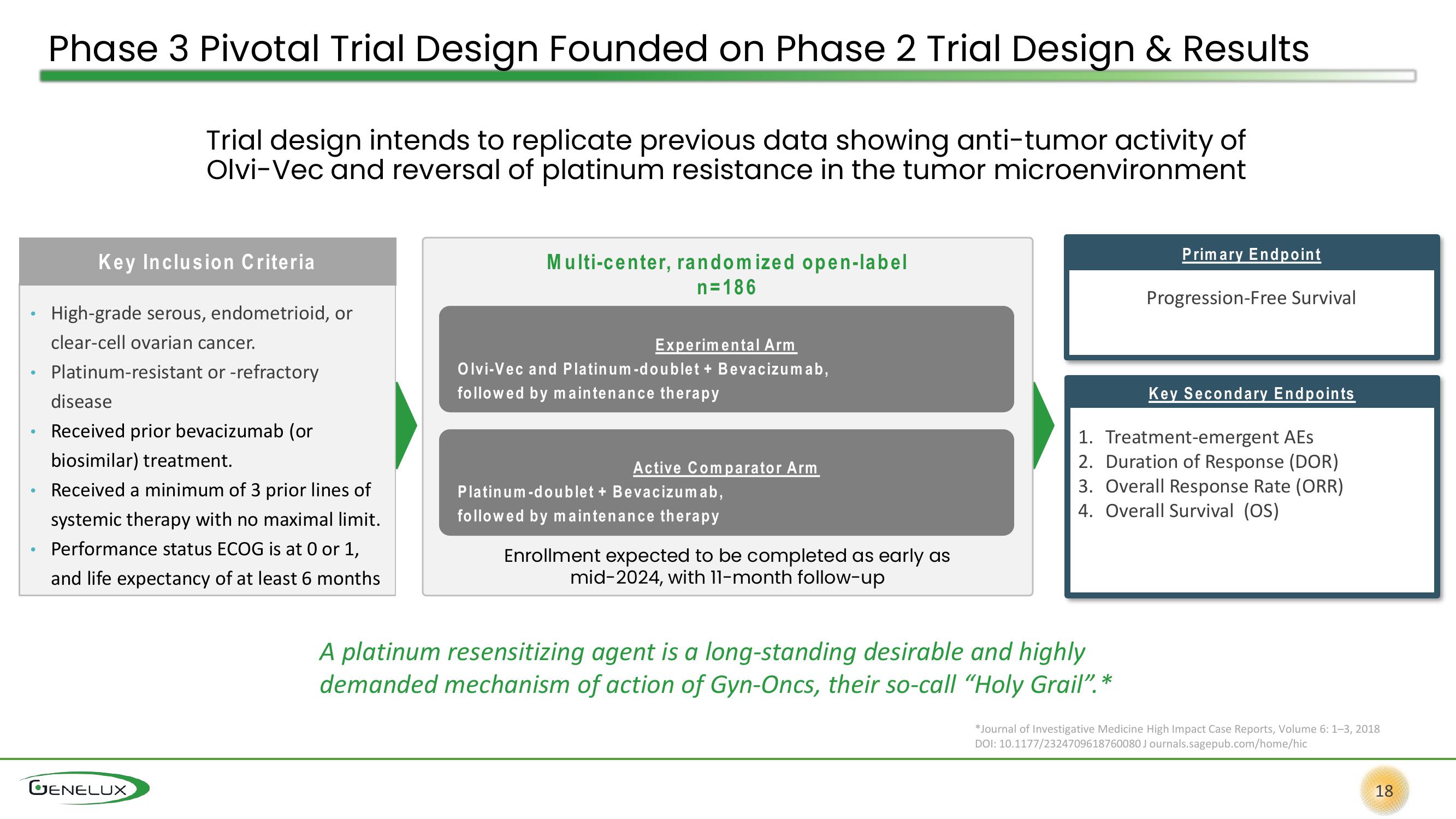
Phase 3 Pivotal Trial Design Founded on Phase 2 Trial Design & Results
Trial design intends to replicate previous data showing anti-tumor activity of
Olvi-Vec and reversal of platinum resistance in the tumor microenvironment
Key Inclusion Criteria
High-grade serous, endometrioid, or
clear-cell ovarian cancer.
Platinum-resistant or -refractory
disease
Received prior bevacizumab (or
biosimilar) treatment.
Received a minimum of 3 prior lines of
systemic therapy with no maximal limit.
Performance status ECOG is at 0 or 1,
and life expectancy of at least 6 months
GENELUX
Multi-center, randomized open-label
n=186
Experimental Arm
Olvi-Vec and Platinum-doublet + Bevacizumab,
followed by maintenance therapy
Active Comparator Arm
Platinum-doublet + Bevacizumab,
followed by maintenance therapy
Enrollment expected to be completed as early as
mid-2024, with 11-month follow-up
Primary Endpoint
Progression-Free Survival
A platinum resensitizing agent is a long-standing desirable and highly
demanded mechanism of action of Gyn-Oncs, their so-call "Holy Grail". *
Key Secondary Endpoints
1. Treatment-emergent AES
2. Duration of Response (DOR)
3. Overall Response Rate (ORR)
4. Overall Survival (OS)
*Journal of Investigative Medicine High Impact Case Reports, Volume 6: 1-3, 2018
DOI: 10.1177/2324709618760080 Journals.sagepub.com/home/hic
18View entire presentation