BioNTech Investor Day Presentation Deck
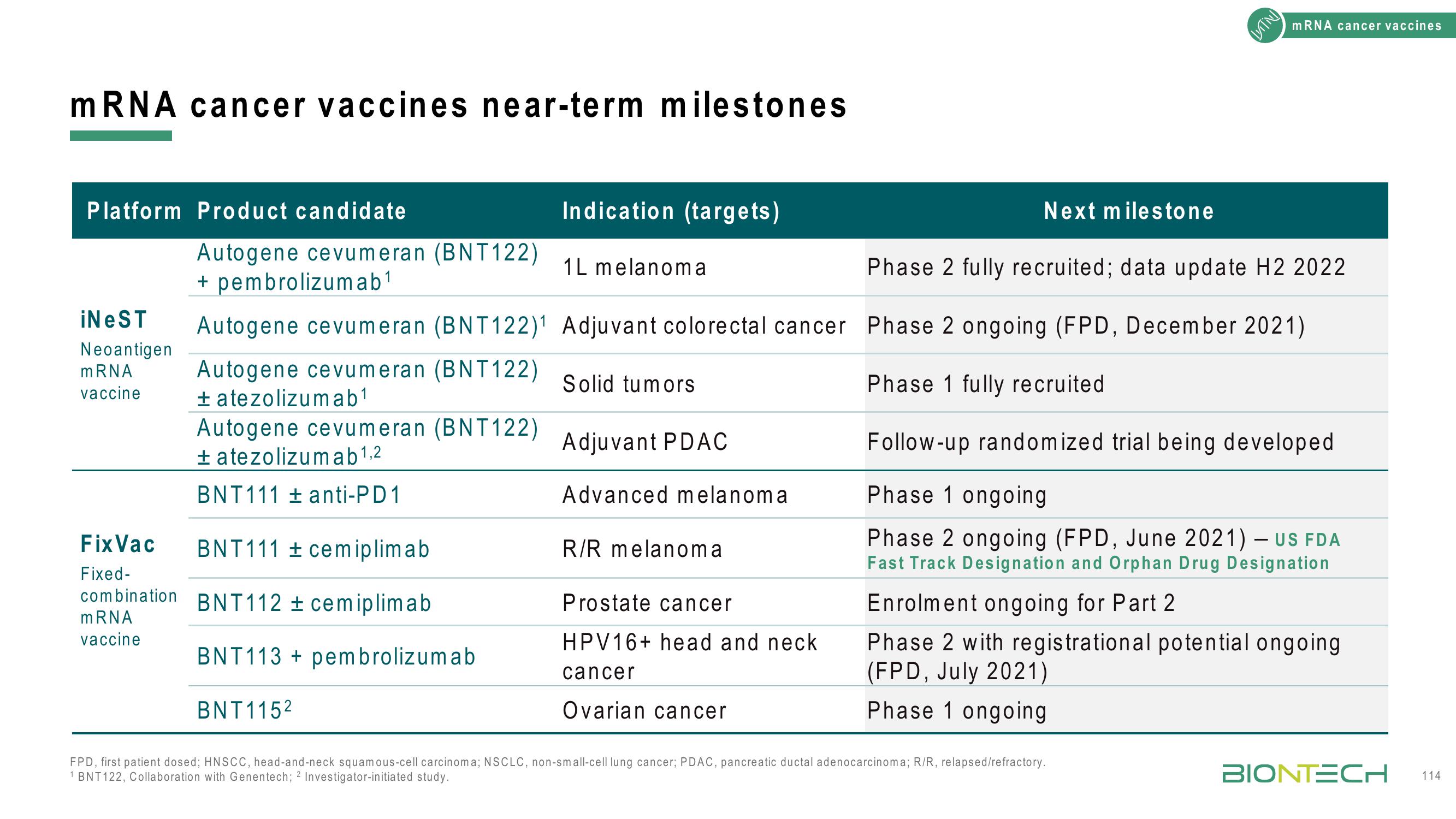
mRNA cancer vaccines near-term milestones
Platform Product candidate
iNeST
Neoantigen
mRNA
vaccine
Fix Vac
Fixed-
combination
mRNA
vaccine
Autogene cevumeran (BNT122)
+ pembrolizumab¹
Autogene cevumeran (BNT122)
± atezolizumab ¹,2
BNT111 ± anti-PD 1
BNT111 cemiplimab
BNT112 ± cemiplimab
BNT113
BNT1152
Indication (targets)
Phase 2 fully recruited; data update H2 2022
Autogene cevumeran (BNT122)1 Adjuvant colorectal cancer Phase 2 ongoing (FPD, December 2021)
Autogene cevumeran (BNT122)
Phase 1 fully recruited
± atezolizumab¹
pembrolizumab
1L melanoma
Solid tumors
Adjuvant PDAC
Advanced melanoma
R/R melanoma
Next milestone
Prostate cancer
HPV16+ head and neck
cancer
Ovarian cancer
NUM
mRNA cancer vaccines
Follow-up randomized trial being developed
Phase 1 ongoing
Phase 2 ongoing (FPD, June 2021) - US FDA
Fast Track Designation and Orphan Drug Designation
FPD, first patient dosed; HNSCC, head-and-neck squamous-cell carcinoma; NSCLC, non-small-cell lung cancer; PDAC, pancreatic ductal adenocarcinoma; R/R, relapsed/refractory.
1 BNT122, Collaboration with Genentech; 2 Investigator-initiated study.
Enrolment ongoing for Part 2
Phase 2 with registrational potential ongoing
(FPD, July 2021)
Phase 1 ongoing
BIONTECH
114View entire presentation