Ocuphire Pharma Results
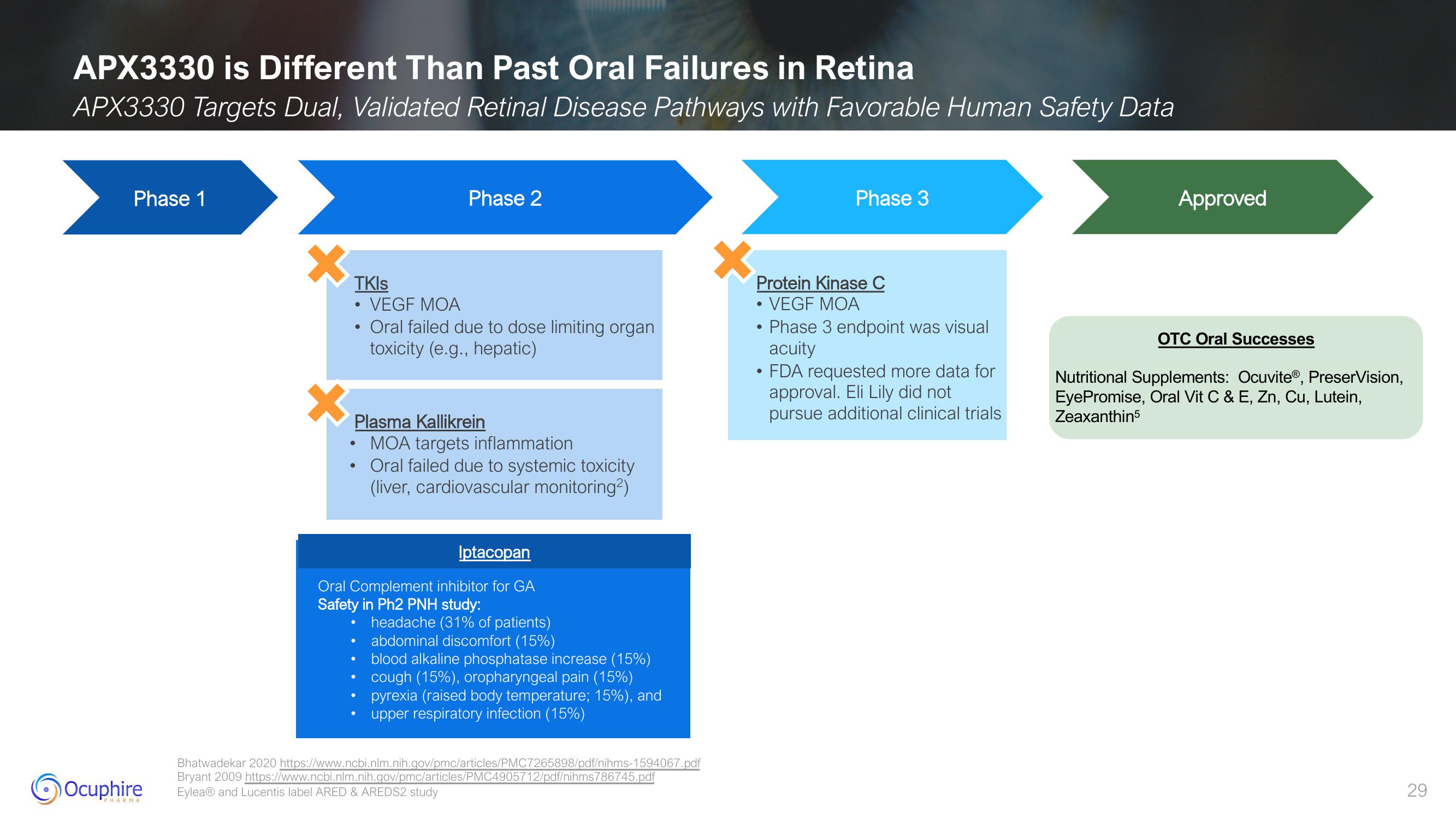
APX3330 is Different Than Past Oral Failures in Retina
APX3330 Targets Dual, Validated Retinal Disease Pathways with Favorable Human Safety Data
Phase 1
TKIs
●
Phase 2
VEGF MOA
Oral failed due to dose limiting organ
toxicity (e.g., hepatic)
Plasma Kallikrein
MOA targets inflammation
Oral failed due to systemic toxicity
(liver, cardiovascular monitoring²)
●
Iptacopan
Oral Complement inhibitor for GA
Safety in Ph2 PNH study:
headache (31% of patients)
abdominal discomfort (15%)
blood alkaline phosphatase increase (15%)
cough (15%), oropharyngeal pain (15%)
pyrexia (raised body temperature; 15%), and
upper respiratory infection (15%)
Bhatwadekar 2020 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7265898/pdf/nihms-1594067.pdf
Bryant 2009 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4905712/pdf/nihms786745.pdf
Ocuphire Eylea® and Lucentis label ARED & AREDS2 study
PHARMA
X
Protein Kinase C
VEGF MOA
●
Phase 3
●
Phase 3 endpoint was visual
acuity
FDA requested more data for
approval. Eli Lily did not
pursue additional clinical trials
Approved
OTC Oral Successes
Nutritional Supplements: Ocuvite®, PreserVision,
EyePromise, Oral Vit C & E, Zn, Cu, Lutein,
Zeaxanthin5
29View entire presentation