Acquisition of APIRx
For personal use only
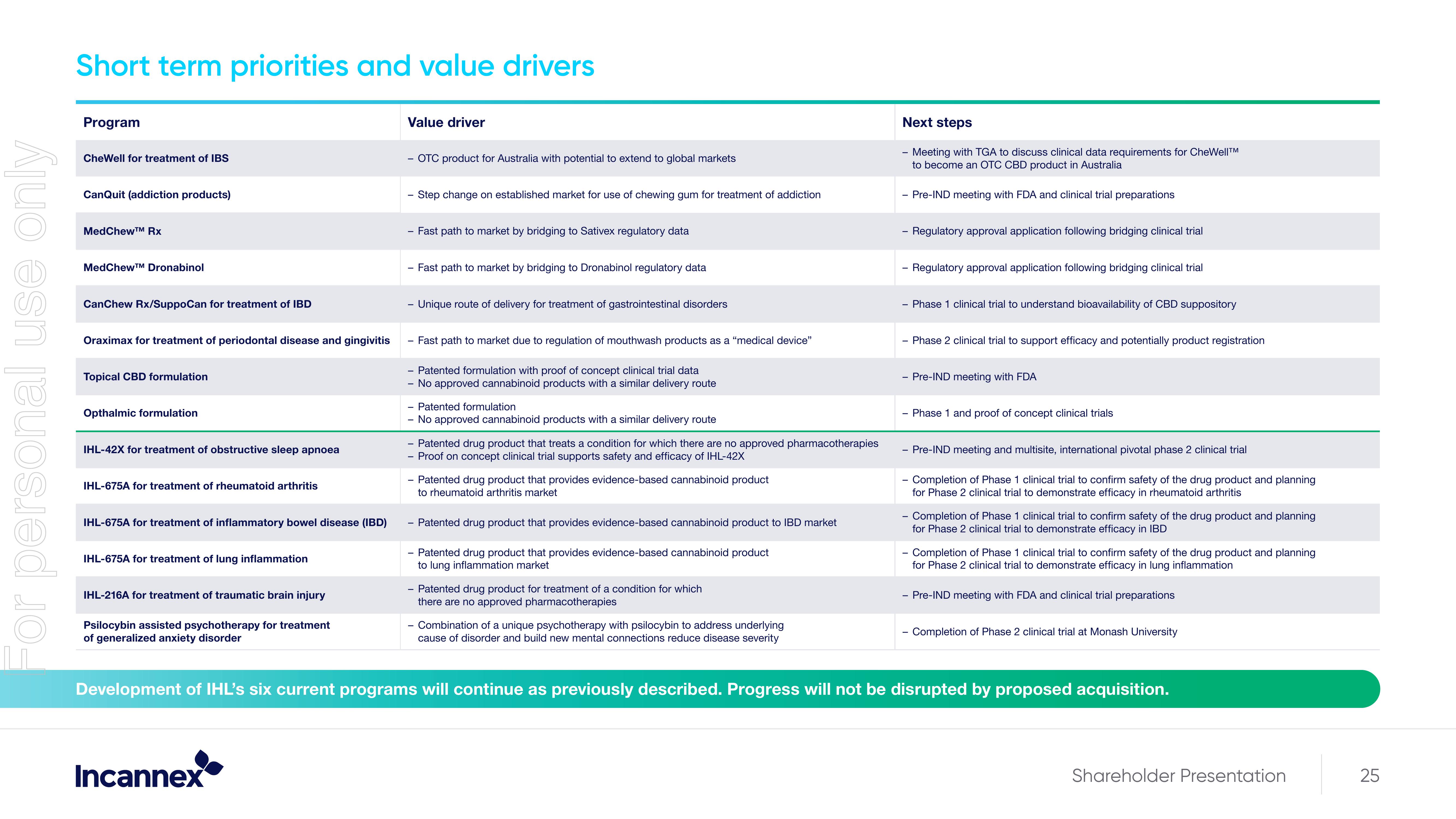
Short term priorities and value drivers
Program
CheWell for treatment of IBS
CanQuit (addiction products)
MedChew™ RX
MedChewTM Dronabinol
CanChew Rx/SuppoCan for treatment of IBD
Oraximax for treatment of periodontal disease and gingivitis
Topical CBD formulation
Opthalmic formulation
IHL-42X for treatment of obstructive sleep apnoea
IHL-675A for treatment of rheumatoid arthritis
IHL-675A for treatment of inflammatory bowel disease (IBD)
IHL-675A for treatment of lung inflammation
IHL-216A for treatment of traumatic brain injury
Psilocybin assisted psychotherapy for treatment
of generalized anxiety disorder
Value driver
Incannex
- OTC product for Australia with potential to extend to global markets
Step change on established market for use of chewing gum for treatment of addiction
- Fast path to market by bridging to Sativex regulatory data
- Fast path to market by bridging to Dronabinol regulatory data
Unique route of delivery for treatment of gastrointestinal disorders
- Fast path to market due to regulation of mouthwash products as a "medical device"
Patented formulation with proof of concept clinical trial data
- No approved cannabinoid products with a similar delivery route
- Patented formulation
No approved cannabinoid products with a similar delivery route
Patented drug product that treats a condition for which there are no approved pharmacotherapies
Proof on concept clinical trial supports safety and efficacy of IHL-42X
- Patented drug product that provides evidence-based cannabinoid product
to rheumatoid arthritis market
Patented drug product that provides evidence-based cannabinoid product to IBD market
- Patented drug product that provides evidence-based cannabinoid product
to lung inflammation market
Patented drug product for treatment of a condition for which
there are no approved pharmacotherapies
- Combination of a unique psychotherapy with psilocybin to address underlying
cause of disorder and build new mental connections reduce disease severity
Next steps
- Meeting with TGA to discuss clinical data requirements for CheWell™
to become an OTC CBD product in Australia
- Pre-IND meeting with FDA and clinical trial preparations
- Regulatory approval application following bridging clinical trial
- Regulatory approval application following bridging clinical trial
- Phase 1 clinical trial to understand bioavailability of CBD suppository
- Phase 2 clinical trial to support efficacy and potentially product registration
- Pre-IND meeting with FDA
- Phase 1 and proof of concept clinical trials
- Pre-IND meeting and multisite, international pivotal phase 2 clinical trial
- Completion of Phase 1 clinical trial to confirm safety of the drug product and planning
for Phase 2 clinical trial to demonstrate efficacy in rheumatoid arthritis
- Completion of Phase 1 clinical trial to confirm safety of the drug product and planning
for Phase 2 clinical trial to demonstrate efficacy in IBD
- Completion of Phase 1 clinical trial to confirm safety of the drug product and planning
for Phase 2 clinical trial to demonstrate efficacy in lung inflammation
- Pre-IND meeting with FDA and clinical trial preparations
- Completion of Phase 2 clinical trial at Monash University
Development of IHL's six current programs will continue as previously described. Progress will not be disrupted by proposed acquisition.
Shareholder Presentation
25View entire presentation