Equillium Results Presentation Deck
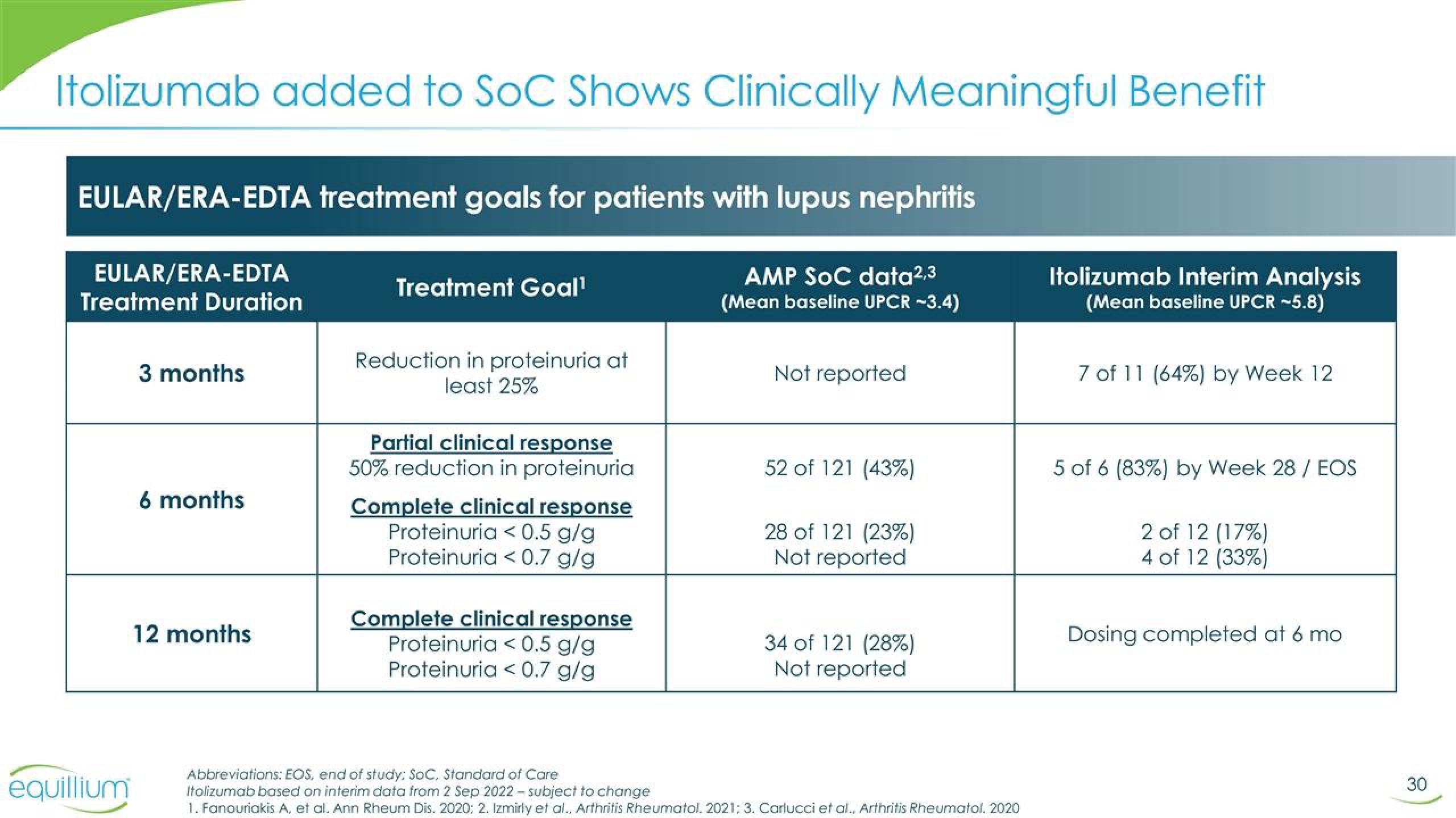
Itolizumab added to SoC Shows Clinically Meaningful Benefit
EULAR/ERA-EDTA treatment goals for patients with lupus nephritis
AMP SOC data2,3
(Mean baseline UPCR -3.4)
EULAR/ERA-EDTA
Treatment Duration
equillium
3 months
6 months
12 months
Treatment Goal¹
Reduction in proteinuria at
least 25%
Partial clinical response
50% reduction in proteinuria
Complete clinical response
Proteinuria < 0.5 g/g
Proteinuria < 0.7 g/g
Complete clinical response
Proteinuria < 0.5 g/g
Proteinuria < 0.7 g/g
Not reported
52 of 121 (43%)
28 of 121 (23%)
Not reported
34 of 121 (28%)
Not reported
Abbreviations: EOS, end of study: SoC, Standard of Care
Itolizumab based on interim data from 2 Sep 2022-subject to change
1. Fanouriakis A, et al. Ann Rheum Dis. 2020; 2. Izmirly et al., Arthritis Rheumatol. 2021; 3. Carlucci et al., Arthritis Rheumatol. 2020
Itolizumab Interim Analysis
(Mean baseline UPCR -5.8)
7 of 11 (64%) by Week 12
5 of 6 (83%) by Week 28/ EOS
2 of 12 (17%)
4 of 12 (33%)
Dosing completed at 6 mo
30View entire presentation