Immix Biopharma Investor Presentation Deck
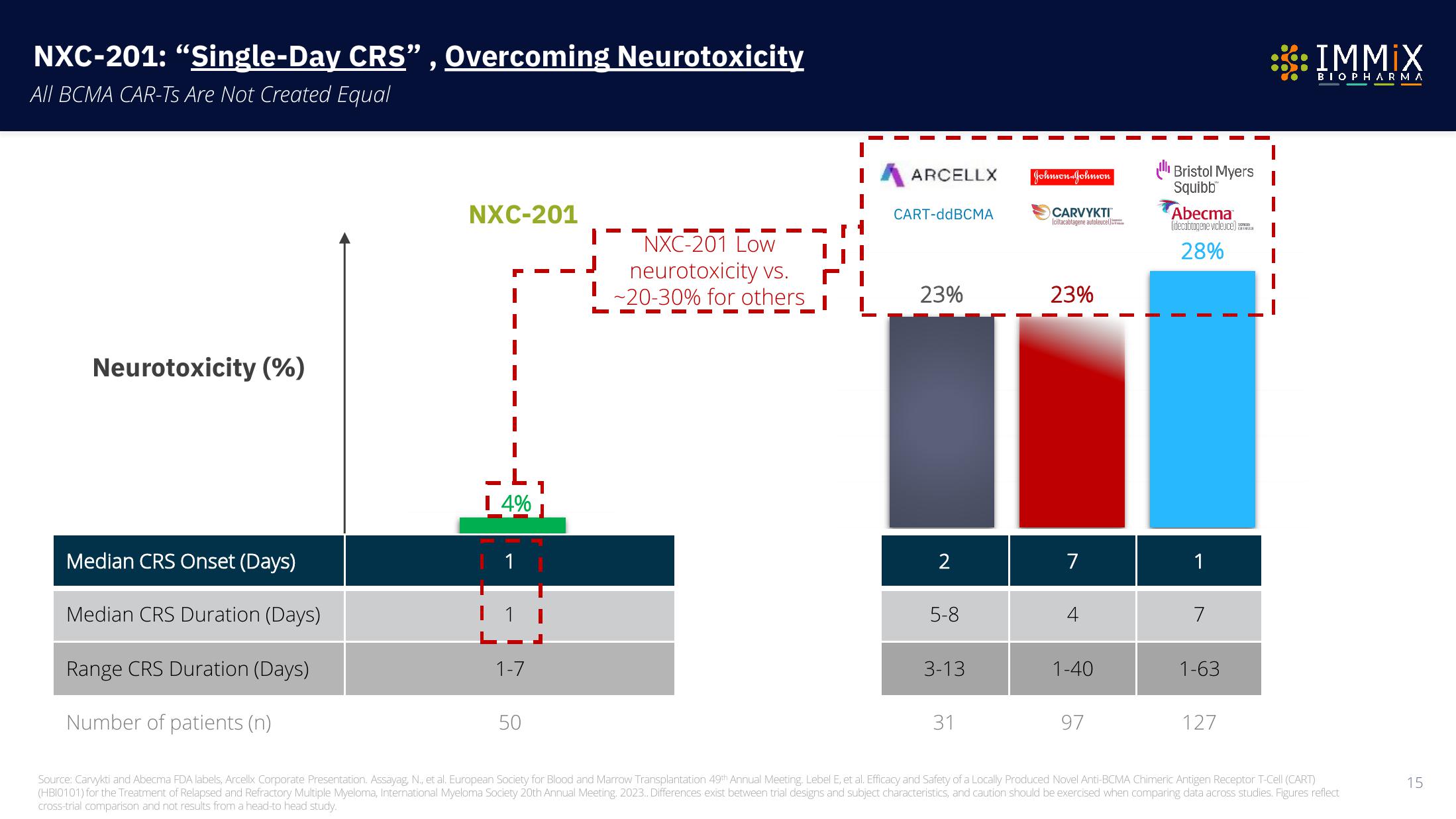
NXC-201: "Single-Day CRS”, Overcoming Neurotoxicity
All BCMA CAR-Ts Are Not Created Equal
Neurotoxicity (%)
Median CRS Onset (Days)
Median CRS Duration (Days)
Range CRS Duration (Days)
Number of patients (n)
NXC-201
I
I
I 4%
1
1-7
50
NXC-201 Low
neurotoxicity vs.
-20-30% for others
ARCELLX
CART-ddBCMA
23%
2
5-8
3-13
31
Johnson Johnson
CARVYKTI
(ciltacabtagene autoleuce)
23%
7
1-40
97
ll Bristol Myers
Squibb
Abecma
(idecabtagene vicleuce)
28%
1
7
1-63
127
●●●
S
IMMIX
BIOPHARMA
Source: Carvykti and Abecma FDA labels, Arcellx Corporate Presentation. Assayag, N., et al. European Society for Blood and Marrow Transplantation 49th Annual Meeting. Lebel E, et al. Efficacy and Safety of a Locally Produced Novel Anti-BCMA Chimeric Antigen Receptor T-Cell (CART)
(HBIO101) for the Treatment of Relapsed and Refractory Multiple Myeloma, International Myeloma Society 20th Annual Meeting, 2023.. Differences exist between trial designs and subject characteristics, and caution should be exercised when comparing data across studies. Figures reflect
cross-trial comparison and not results from a head-to head study.
15View entire presentation