Kymera Investor Presentation Deck
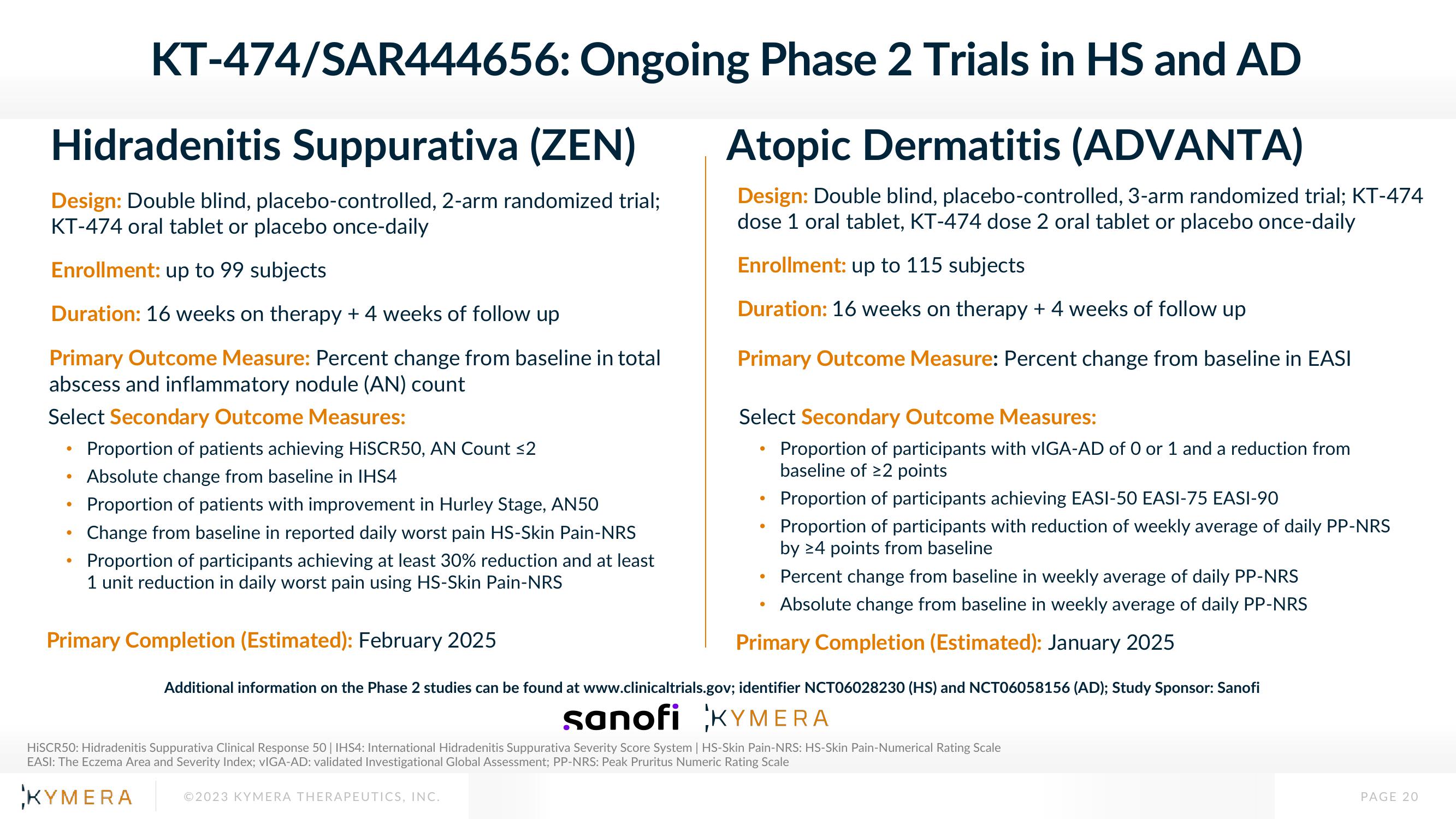
KT-474/SAR444656:
Hidradenitis Suppurativa (ZEN)
Design: Double blind, placebo-controlled, 2-arm randomized trial;
KT-474 oral tablet or placebo once-daily
Enrollment: up to 99 subjects
Duration: 16 weeks on therapy + 4 weeks of follow up
Primary Outcome Measure: Percent change from baseline in total
abscess and inflammatory nodule (AN) count
Select Secondary Outcome Measures:
●
●
●
●
●
Ongoing Phase 2 Trials in HS and AD
Atopic Dermatitis (ADVANTA)
Design: Double blind, placebo-controlled, 3-arm randomized trial; KT-474
dose 1 oral tablet, KT-474 dose 2 oral tablet or placebo once-daily
Enrollment: up to 115 subjects
Duration: 16 weeks on therapy + 4 weeks of follow up
Primary Outcome Measure: Percent change from baseline in EASI
Proportion of patients achieving HiSCR50, AN Count ≤2
Absolute change from baseline in IHS4
Proportion of patients with improvement in Hurley Stage, AN50
Change from baseline in reported daily worst pain HS-Skin Pain-NRS
Proportion of participants achieving at least 30% reduction and at least
1 unit reduction in daily worst pain using HS-Skin Pain-NRS
Primary Completion (Estimated): February 2025
Select Secondary Outcome Measures:
Proportion of participants with vIGA-AD of 0 or 1 and a reduction from
baseline of 22 points
Proportion of participants achieving EASI-50 EASI-75 EASI-90
Proportion of participants with reduction of weekly average of daily PP-NRS
by 24 points from baseline
●
●
Percent change from baseline in weekly average of daily PP-NRS
Absolute change from baseline in weekly average of daily PP-NRS
Primary Completion (Estimated): January 2025
Additional information on the Phase 2 studies can be found at www.clinical trials.gov; identifier NCT06028230 (HS) and NCT06058156 (AD); Study Sponsor: Sanofi
sanofi KYMERA
HISCR50: Hidradenitis Suppurativa Clinical Response 50 | IHS4: International Hidradenitis Suppurativa Severity Score System | HS-Skin Pain-NRS: HS-Skin Pain-Numerical Rating Scale
EASI: The Eczema Area and Severity Index; vIGA-AD: validated Investigational Global Assessment; PP-NRS: Peak Pruritus Numeric Rating Scale
KYMERA ©2023 KYMERA THERAPEUTICS, INC.
PAGE 20View entire presentation