BioAtla Investor Presentation Deck
BA3071 (CAB-CTLA-4)
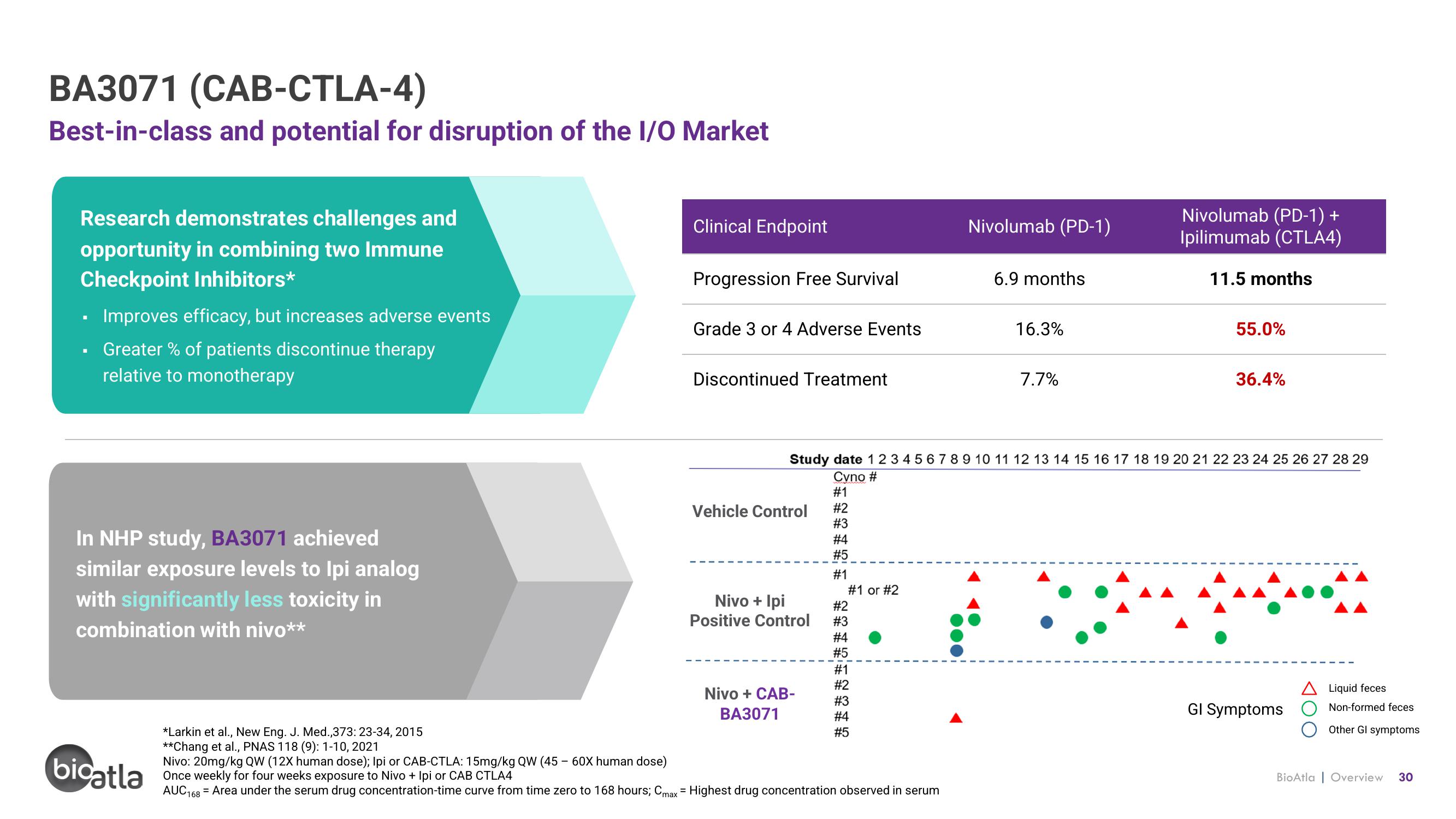
Best-in-class and potential for disruption of the I/O Market
Research demonstrates challenges and
opportunity in combining two Immune
Checkpoint Inhibitors*
I
-
Improves efficacy, but increases adverse events
Greater % of patients discontinue therapy
relative to monotherapy
In NHP study, BA3071 achieved
similar exposure levels to Ipi analog
with significantly less toxicity in
combination with nivo**
bicatla
Clinical Endpoint
Progression Free Survival
Grade 3 or 4 Adverse Events
Discontinued Treatment
Vehicle Control
Nivo + Ipi
Positive Control
Nivo + CAB-
BA3071
#1
#2
#3
#4
#5
#1
#2
#3
######
Study date 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29
Cvno #
#4
#1 or #2
#5
#1
#2
#3
#4
*Larkin et al., New Eng. J. Med.,373: 23-34, 2015
**Chang et al., PNAS 118 (9): 1-10, 2021
Nivo: 20mg/kg QW (12X human dose); Ipi or CAB-CTLA: 15mg/kg QW (45-60X human dose)
Once weekly for four weeks exposure to Nivo + Ipi or CAB CTLA4
AUC168 = Area under the serum drug concentration-time curve from time zero to 168 hours; Cmax = Highest drug concentration observed in serum
#5
Nivolumab (PD-1)
●●●
6.9 months
16.3%
7.7%
Nivolumab (PD-1) +
Ipilimumab (CTLA4)
11.5 months
55.0%
36.4%
GI Symptoms
A Liquid feces
Non-formed feces
Other Gl symptoms
BioAtla| Overview 30View entire presentation