argenx SE Investor Day Presentation Deck
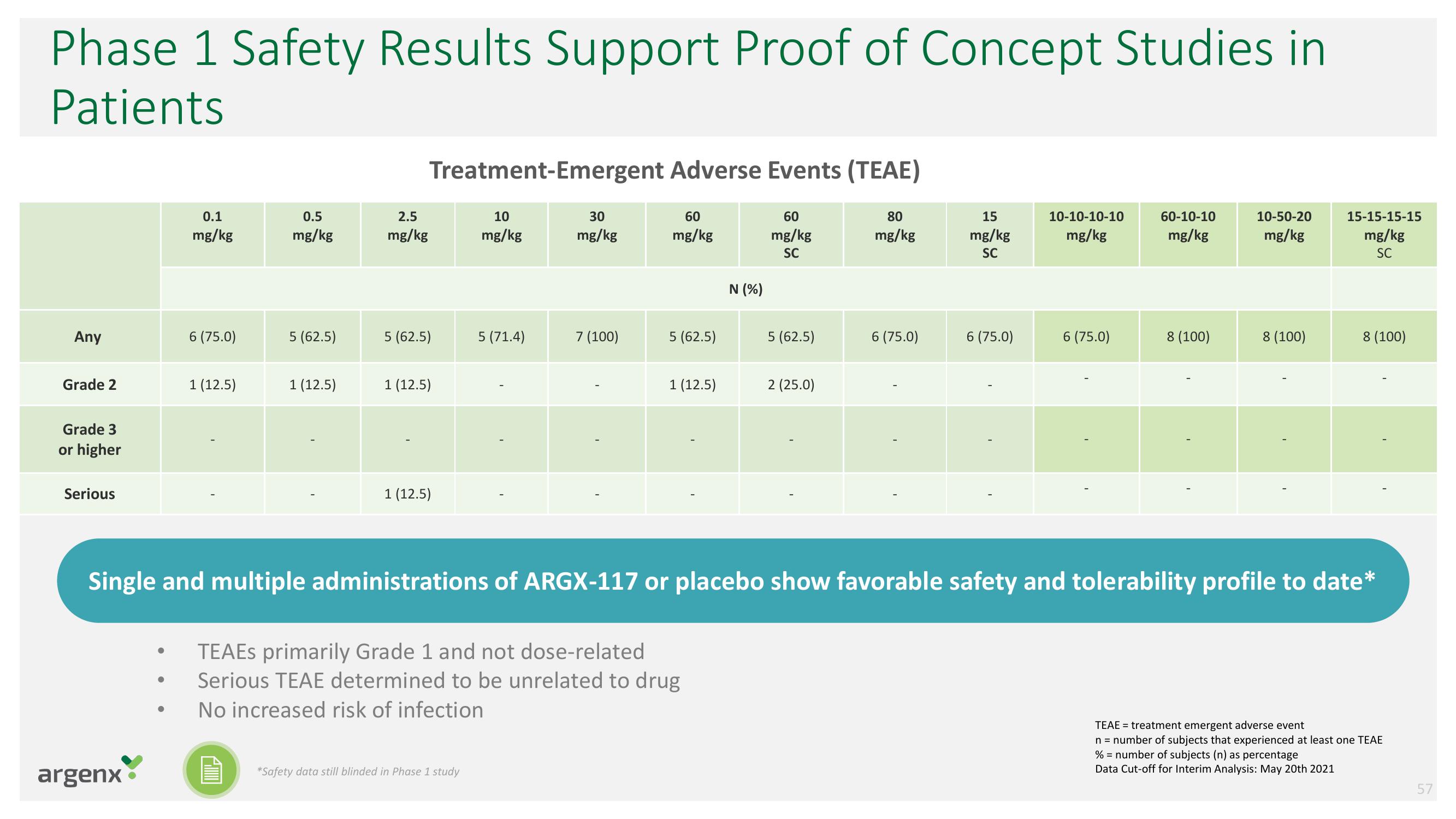
Phase 1 Safety Results Support Proof of Concept Studies in
Patients
Any
Grade 2
Grade 3
or higher
Serious
argenx
●
●
0.1
mg/kg
●
6 (75.0)
1 (12.5)
0.5
mg/kg
5 (62.5)
1 (12.5)
2.5
mg/kg
Treatment-Emergent Adverse Events (TEAE)
5 (62.5)
1 (12.5)
1 (12.5)
10
mg/kg
5 (71.4)
*Safety data still blinded in Phase 1 study
30
mg/kg
7 (100)
60
mg/kg
5 (62.5)
1 (12.5)
TEAEs primarily Grade 1 and not dose-related
Serious TEAE determined to be unrelated to drug
No increased risk of infection
N (%)
60
mg/kg
SC
5 (62.5)
2 (25.0)
80
mg/kg
6 (75.0)
15
mg/kg
SC
6 (75.0)
10-10-10-10
mg/kg
6 (75.0)
60-10-10
mg/kg
Single and multiple administrations of ARGX-117 or placebo show favorable safety and tolerability profile to date*
8 (100)
10-50-20
mg/kg
8 (100)
15-15-15-15
mg/kg
SC
8 (100)
TEAE = treatment emergent adverse event
n = number of subjects that experienced at least one TEAE
% = number of subjects (n) as percentage
Data Cut-off for Interim Analysis: May 20th 2021
57View entire presentation