Nuvectis Pharma Investor Presentation Deck
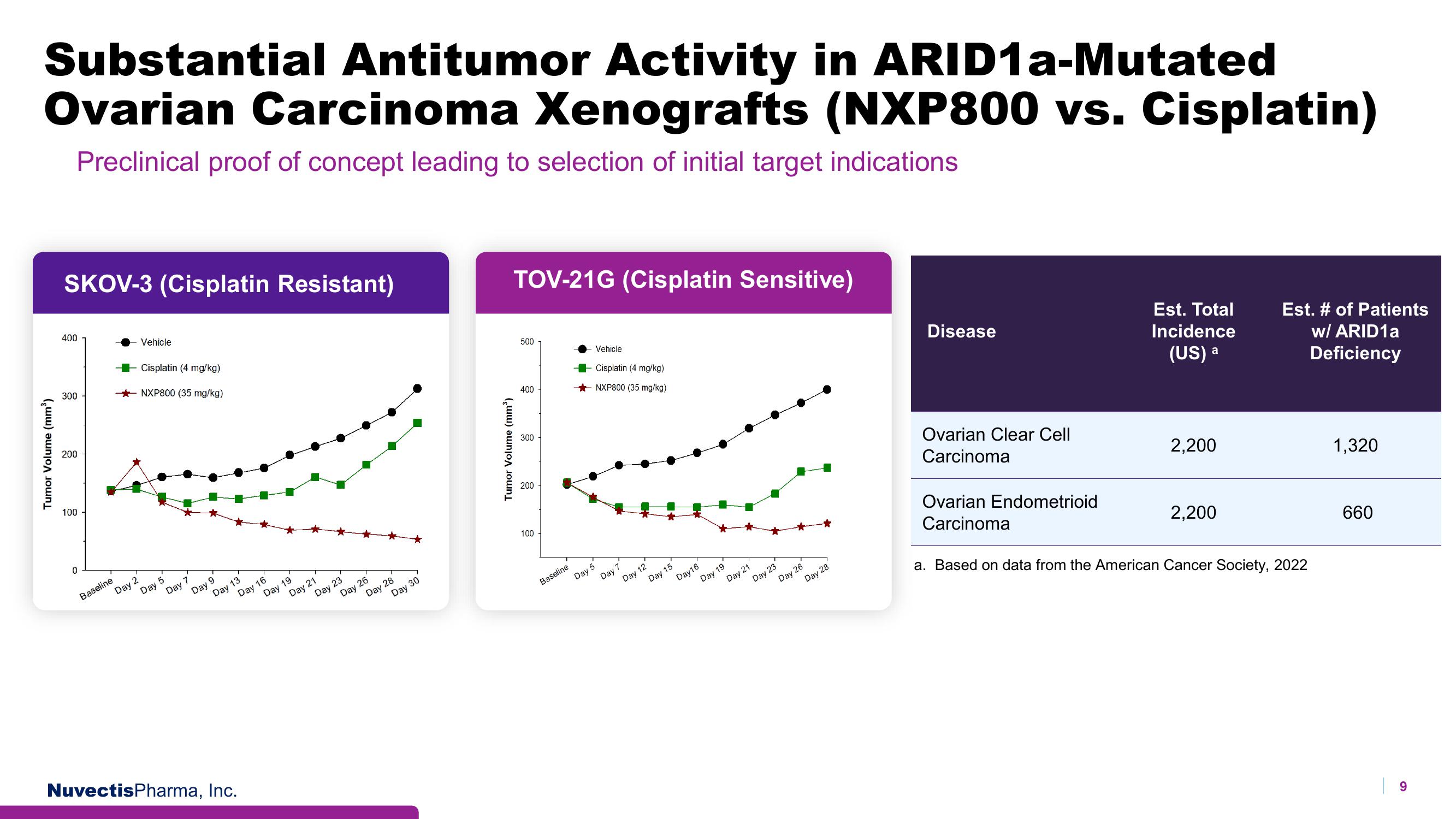
Substantial Antitumor Activity in ARID1a-Mutated
Ovarian Carcinoma Xenografts (NXP800 vs. Cisplatin)
Preclinical proof of concept leading to selection of initial target indications
Tumor Volume (mm³)
SKOV-3 (Cisplatin Resistant)
400
300
200
100
0
Day 2
Baseline
Vehicle
Cisplatin (4 mg/kg)
NXP800 (35 mg/kg)
Day 5
Day 7
Day 9
Day 13
Day 16
NuvectisPharma, Inc.
Day 19
Day 21
Day 28
Day 23
Day 26
Day 30
Tumor Volume (mm³)
TOV-21G (Cisplatin Sensitive)
500
400
300
200
100
Baseline
Vehicle
Cisplatin (4 mg/kg)
NXP800 (35 mg/kg)
Day 5
Day 7
Day 15
Day 12
Day16
Day 19
Day 21
Day 23
Day 26
Day 28
Disease
Ovarian Clear Cell
Carcinoma
Ovarian Endometrioid
Carcinoma
Est. Total
Incidence
(US) a
2,200
2,200
Est. # of Patients
w/ ARID1a
Deficiency
a. Based on data from the American Cancer Society, 2022
1,320
660
9View entire presentation