AstraZeneca Investor Day Presentation Deck
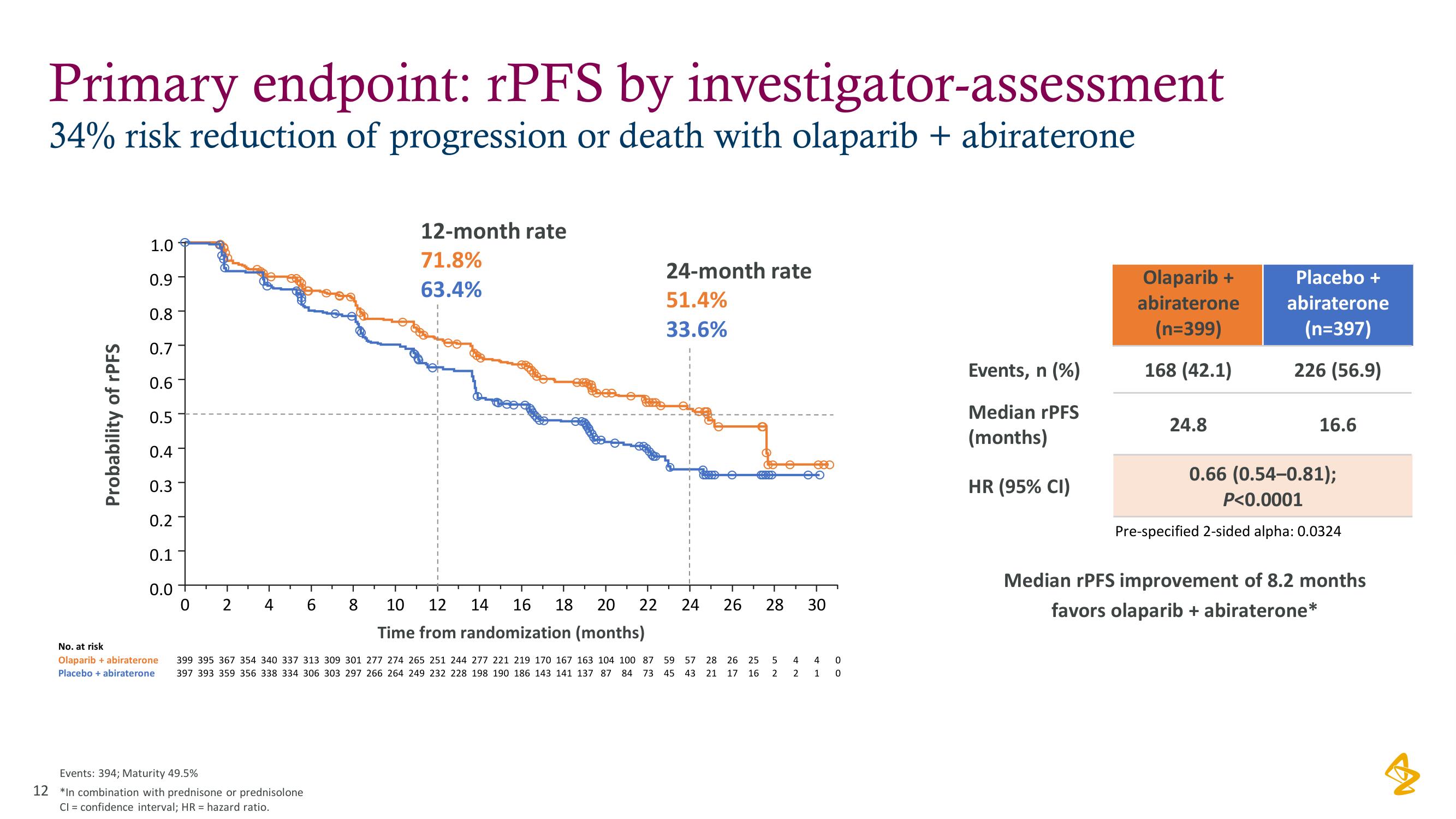
Primary endpoint: rPFS by investigator-assessment
34% risk reduction of progression or death with olaparib + abiraterone
Probability of rPFS
1.0
0.9
0.8-
0.7
0.6
0.5
0.4
0.3 -
0.2
0.1
0.0
No. at risk
Olaparib + abiraterone
Placebo + abiraterone
T
0
T
10 12
14 16 18 20
Time from randomization (months)
399 395 367 354 340 337 313 309 301 277 274 265 251 244 277 221 219 170 167 163 104 100 87 59 57 28 26 25 5
397 393 359 356 338 334 306 303 297 266 264 249 232 228 198 190 186 143 141 137 87 84 73 45 43 21 17 16 2
N
1
4 6
T
8
Events: 394; Maturity 49.5%
12 *In combination with prednisone or prednisolone
CI= confidence interval; HR = hazard ratio.
12-month rate
71.8%
63.4%
T
24-month rate
51.4%
33.6%
22
24
1
26 28
4
2
30
4
1
ос
Events, n (%)
Median rPFS
(months)
HR (95% CI)
Olaparib +
abiraterone
(n=399)
168 (42.1)
24.8
Placebo +
abiraterone
(n=397)
226 (56.9)
16.6
0.66 (0.54-0.81);
P<0.0001
Pre-specified 2-sided alpha: 0.0324
Median rPFS improvement of 8.2 months
favors olaparib + abiraterone*
BView entire presentation