ATAI Investor Presentation Deck
SUMMARY
OWNERSHIP 51.9%¹
PRODUCT
PHARMA-
COLOGY
PRODUCT
FEATURES
INDICATIONS
CURRENT
STATUS
INTELLECTUAL
PROPERTY
HIGHLIGHT
(2R, 3S)-2-amino-3-hydroxy-3-pyridin-4-yl-1-
pyrrolidin-1-yl-propan-1-one(L)-(+) tartrate salt
oral capsules (RL-007)
Cholinergic, glutamatergic and
GABA-B receptor modulator
No drug-related serious adverse events in over
500 study subject exposures, pro-cognitive
effects demonstrated in two Phase 1 and two
Phase 2 trials
Primary: Cognitive Impairment Associated with
Schizophrenia
Potential: Autism, Alzheimer's dementia
Phase 2a trial completed in H2'21
Issued composition of matter, formulation and
method of use patents
Previous Phase 2 showed pro-cognitive
potential of RL-007 in 180 patients with
diabetic peripheral neuropathic pain
22
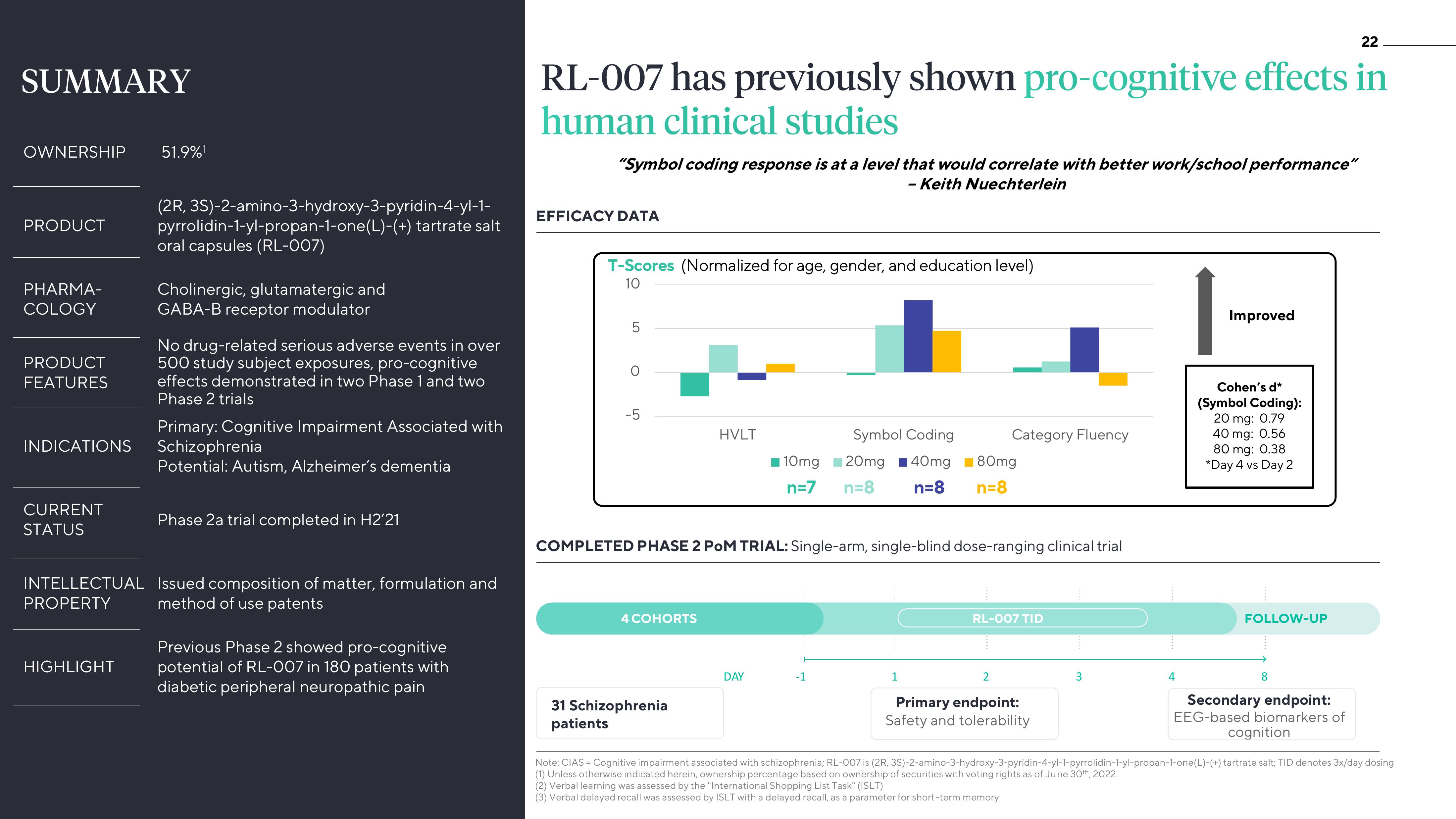
RL-007 has previously shown pro-cognitive effects in
human clinical studies
"Symbol coding response is at a level that would correlate with better work/school performance"
- Keith Nuechterlein
EFFICACY DATA
T-Scores (Normalized for age, gender, and education level)
10
5
O
-5
4 COHORTS
HVLT
31 Schizophrenia
patients
COMPLETED PHASE 2 POM TRIAL: Single-arm, single-blind dose-ranging clinical trial
Symbol Coding
10mg 20mg 40mg ■80mg
n=7 n=8 n=8 n=8
DAY
-1
Category Fluency
1
RL-007 TID
2
Primary endpoint:
Safety and tolerability
3
4
Improved
Cohen's d*
(Symbol Coding):
20 mg: 0.79
40 mg: 0.56
80 mg: 0.38
*Day 4 vs Day 2
Note: CIAS = Cognitive impairment associated with schizophrenia; RL-007 is (2R, 3S)-2-amino-3-hydroxy-3-pyridin-4-yl-1-pyrrolidin-1-yl-propan-1-one(L)-(+)
(1) Unless otherwise indicated herein, ownership percentage based on ownership of securities with voting rights as of June 30th, 2022.
(2) Verbal learning was assessed by the "International Shopping List Task" (ISLT)
(3) Verbal delayed recall was assessed by ISLT with a delayed recall, as a parameter for short-term memory
FOLLOW-UP
8
Secondary endpoint:
EEG-based biomarkers of
cognition
tartrate salt; TID denotes 3x/day dosingView entire presentation