BioAtla Investor Presentation Deck
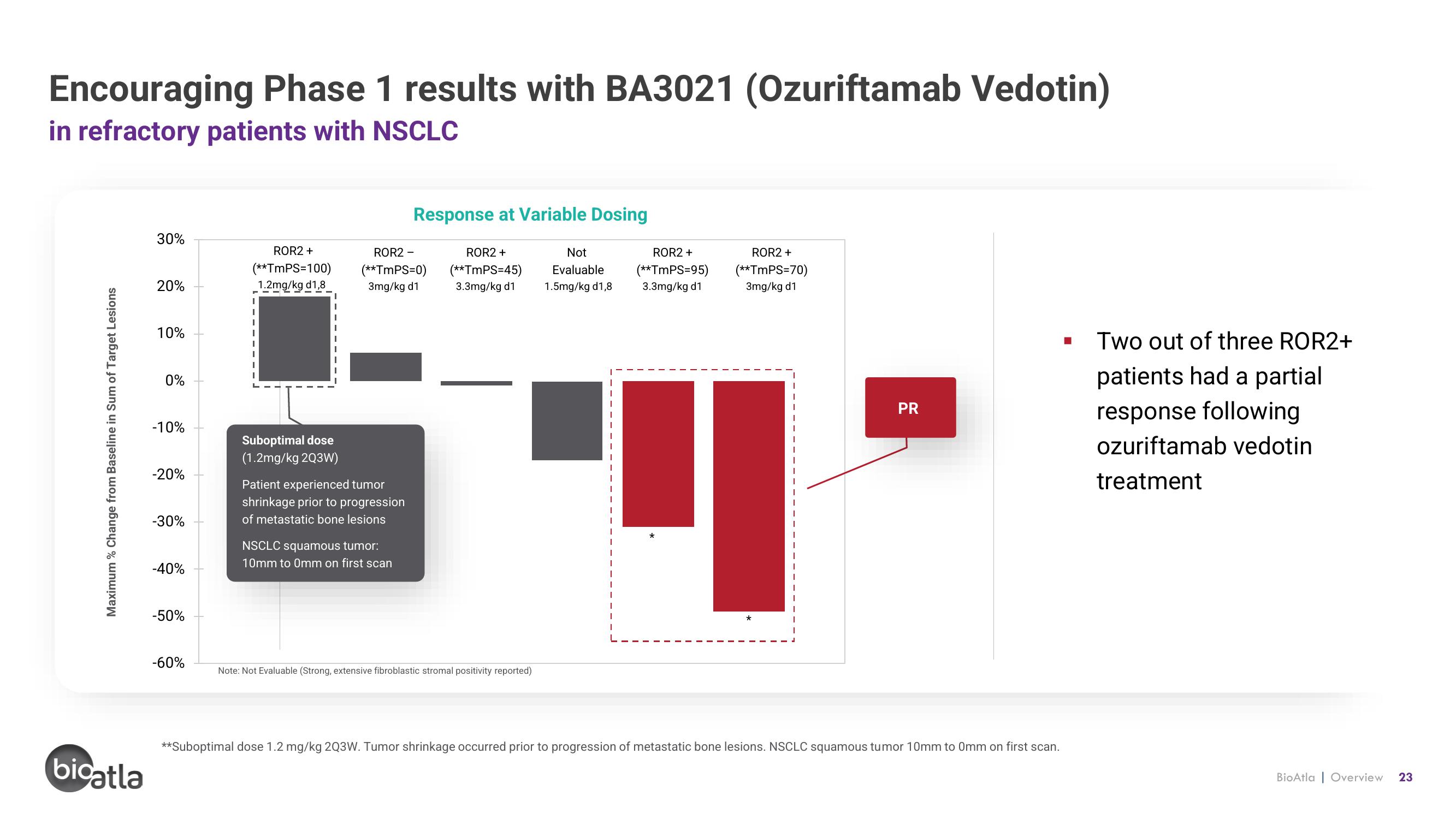
Encouraging Phase 1 results with BA3021 (Ozuriftamab Vedotin)
in refractory patients with NSCLC
Maximum % Change from Baseline in Sum of Target Lesions
bicatla
30%
20%
10%
0%
-10%
-20%
-30%
-40%
-50%
-60%
ROR2 +
(**TmPS=100)
1.2mg/kg d1,8
I
I
I
I
I
Suboptimal dose
(1.2mg/kg 2Q3W)
ROR2 -
(**TmPS=0)
3mg/kg d1
Patient experienced tumor
shrinkage prior to progression
of metastatic bone lesions
Response at Variable Dosing
ROR2 +
(**TmPS=45)
3.3mg/kg d1
NSCLC squamous tumor:
10mm to 0mm on first scan
Not
Evaluable
1.5mg/kg d1,8
Note: Not Evaluable (Strong, extensive fibroblastic stromal positivity reported)
ROR2 +
(**TmPS=95)
3.3mg/kg d1
¶
I
I
ROR2 +
(**TmPS=70)
3mg/kg d1
*
PR
**Suboptimal dose 1.2 mg/kg 2Q3W. Tumor shrinkage occurred prior to progression of metastatic bone lesions. NSCLC squamous tumor 10mm to 0mm on first scan.
■
Two out of three ROR2+
patients had a partial
response following
ozuriftamab vedotin
treatment
BioAtla| Overview 23View entire presentation