Calliditas Therapeutics IPO Presentation Deck
Nefecon was generally well-tolerated in NEFIGAN
Solicited
GCS AES
Overview of
all AEs
calliditas
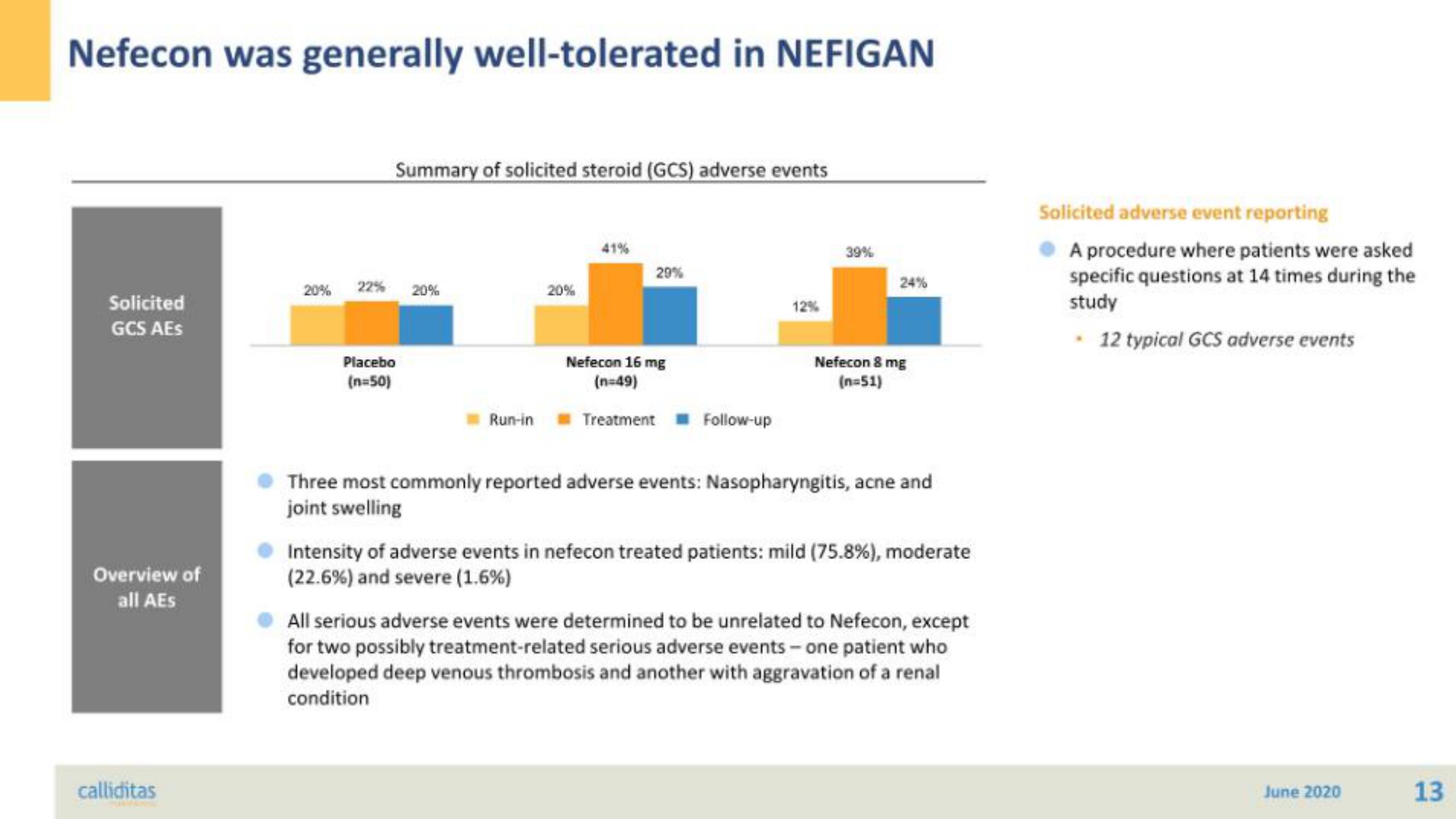
Summary of solicited steroid (GCS) adverse events
20% 22% 20%
Placebo
(n=50)
Run-in
20%
41%
29%
Nefecon 16 mg
(n=49)
Treatment Follow-up
39%
24%
Nefecon 8 mg
(n=51)
Three most commonly reported adverse events: Nasopharyngitis, acne and
joint swelling
Intensity of adverse events in nefecon treated patients: mild (75.8%), moderate
(22.6%) and severe (1.6%)
All serious adverse events were determined to be unrelated to Nefecon, except
for two possibly treatment-related serious adverse events - one patient who
developed deep venous thrombosis and another with aggravation of a renal
condition
Solicited adverse event reporting
A procedure where patients were asked
specific questions at 14 times during the
study
- 12 typical GCS adverse events
June 2020
13View entire presentation