Ocuphire Pharma Investor Day Presentation Deck
DR
DME
40
Summary of APX3330 Program
L
J
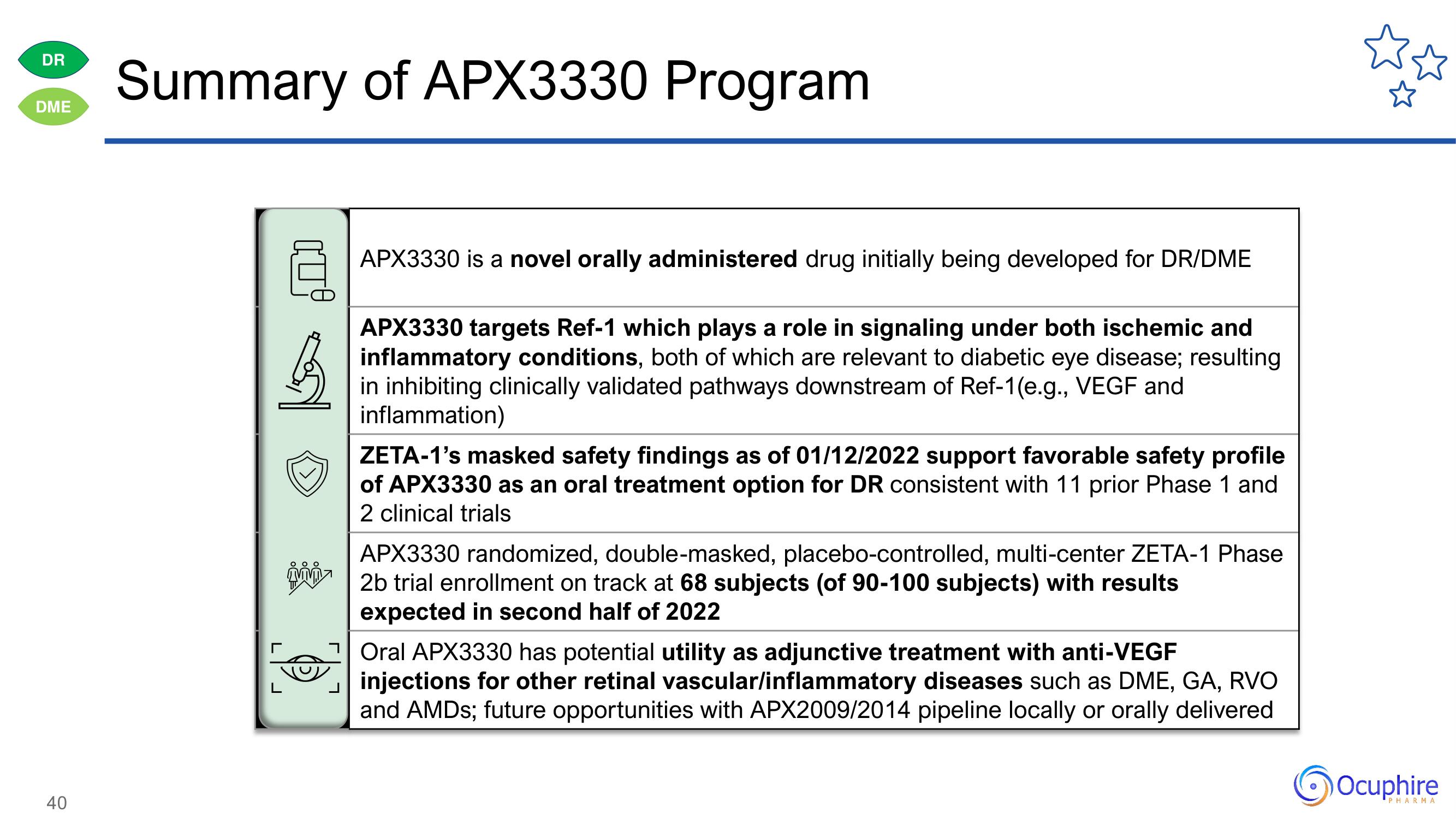
APX3330 is a novel orally administered drug initially being developed for DR/DME
APX3330 targets Ref-1 which plays a role in signaling under both ischemic and
inflammatory conditions, both of which are relevant to diabetic eye disease; resulting
in inhibiting clinically validated pathways downstream of Ref-1(e.g., VEGF and
inflammation)
ZETA-1's masked safety findings as of 01/12/2022 support favorable safety profile
of APX3330 as an oral treatment option for DR consistent with 11 prior Phase 1 and
2 clinical trials
APX3330 randomized, double-masked, placebo-controlled, multi-center ZETA-1 Phase
2b trial enrollment on track at 68 subjects (of 90-100 subjects) with results
expected in second half of 2022
Oral APX3330 has potential utility as adjunctive treatment with anti-VEGF
injections for other retinal vascular/inflammatory diseases such as DME, GA, RVO
and AMDs; future opportunities with APX2009/2014 pipeline locally or orally delivered
☆☆
Ocuphire
PHARMAView entire presentation