Centessa IPO Presentation Deck
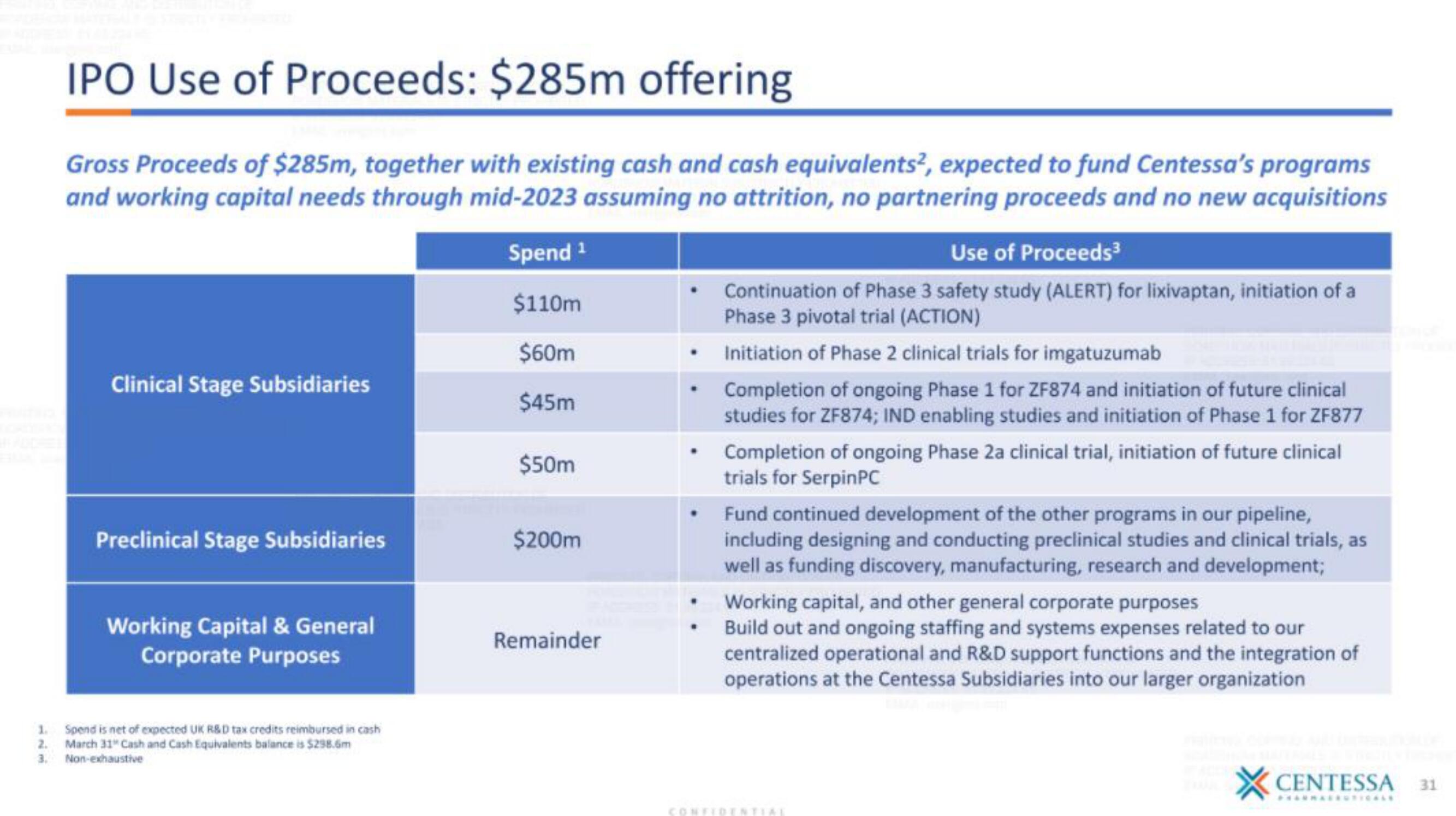
IPO Use of Proceeds: $285m offering
Gross Proceeds of $285m, together with existing cash and cash equivalents², expected to fund Centessa's programs
and working capital needs through mid-2023 assuming no attrition, no partnering proceeds and no new acquisitions
1.
2.
Clinical Stage Subsidiaries
Preclinical Stage Subsidiaries
Working Capital & General
Corporate Purposes
Spend is net of expected UK R&D tax credits reimbursed in cash
March 31" Cash and Cash Equivalents balance is $298.6m
3. Non-exhaustive
Spend ¹
$110m
$60m
$45m
$50m
$200m
Remainder
.
Use of Proceeds³
Continuation of Phase 3 safety study (ALERT) for lixivaptan, initiation of a
Phase 3 pivotal trial (ACTION)
Initiation of Phase 2 clinical trials for imgatuzumab
Completion of ongoing Phase 1 for ZF874 and initiation of future clinical
studies for ZF874; IND enabling studies and initiation of Phase 1 for ZF877
Completion of ongoing Phase 2a clinical trial, initiation of future clinical
trials for SerpinPC
Fund continued development of the other programs in our pipeline,
including designing and conducting preclinical studies and clinical trials, as
well as funding discovery, manufacturing, research and development;
Working capital, and other general corporate purposes
Build out and ongoing staffing and systems expenses related to our
centralized operational and R&D support functions and the integration of
operations at the Centessa Subsidiaries into our larger organization
CENTESSA
31View entire presentation