Aravive Investor Presentation Deck
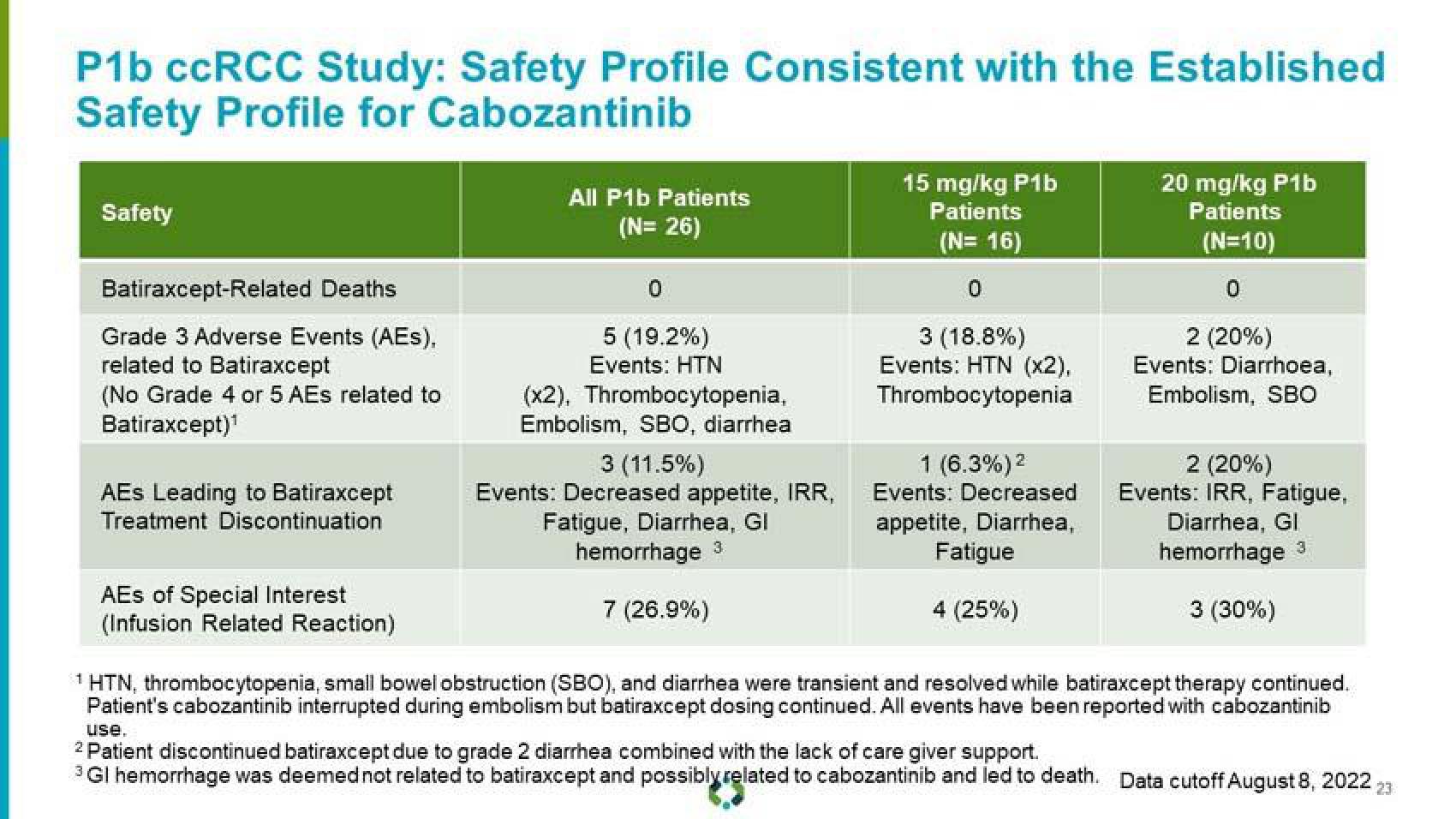
P1b ccRCC Study: Safety Profile Consistent with the Established
Safety Profile for Cabozantinib
Safety
Batiraxcept-Related Deaths
Grade 3 Adverse Events (AES),
related to Batiraxcept
(No Grade 4 or 5 AEs related to
Batiraxcept)¹
AEs Leading to Batiraxcept
Treatment Discontinuation
AES of Special Interest
(Infusion Related Reaction)
All P1b Patients
(N= 26)
5 (19.2%)
Events: HTN
(x2), Thrombocytopenia,
Embolism, SBO, diarrhea
3 (11.5%)
Events: Decreased appetite, IRR,
Fatigue, Diarrhea, Gl
hemorrhage 3
7 (26.9%)
15 mg/kg P1b
Patients
(N=16)
3 (18.8%)
Events: HTN (x2),
Thrombocytopenia
1 (6.3%) ²
Events: Decreased
appetite, Diarrhea,
Fatigue
4 (25%)
20 mg/kg P1b
Patients
(N=10)
2 (20%)
Events: Diarrhoea,
Embolism, SBO
2 (20%)
Events: IRR, Fatigue,
Diarrhea, GI
hemorrhage 3
3 (30%)
¹ HTN, thrombocytopenia, small bowel obstruction (SBO), and diarrhea were transient and resolved while batiraxcept therapy continued.
Patient's cabozantinib interrupted during embolism but batiraxcept dosing continued. All events have been reported with cabozantinib
use.
Patient discontinued batiraxcept due to grade 2 diarrhea combined with the lack of care giver support.
3 GI hemorrhage was deemed not related to batiraxcept and possibly related to cabozantinib and led to death. Data cutoff August 8, 2022 23View entire presentation