Aravive Investor Presentation Deck
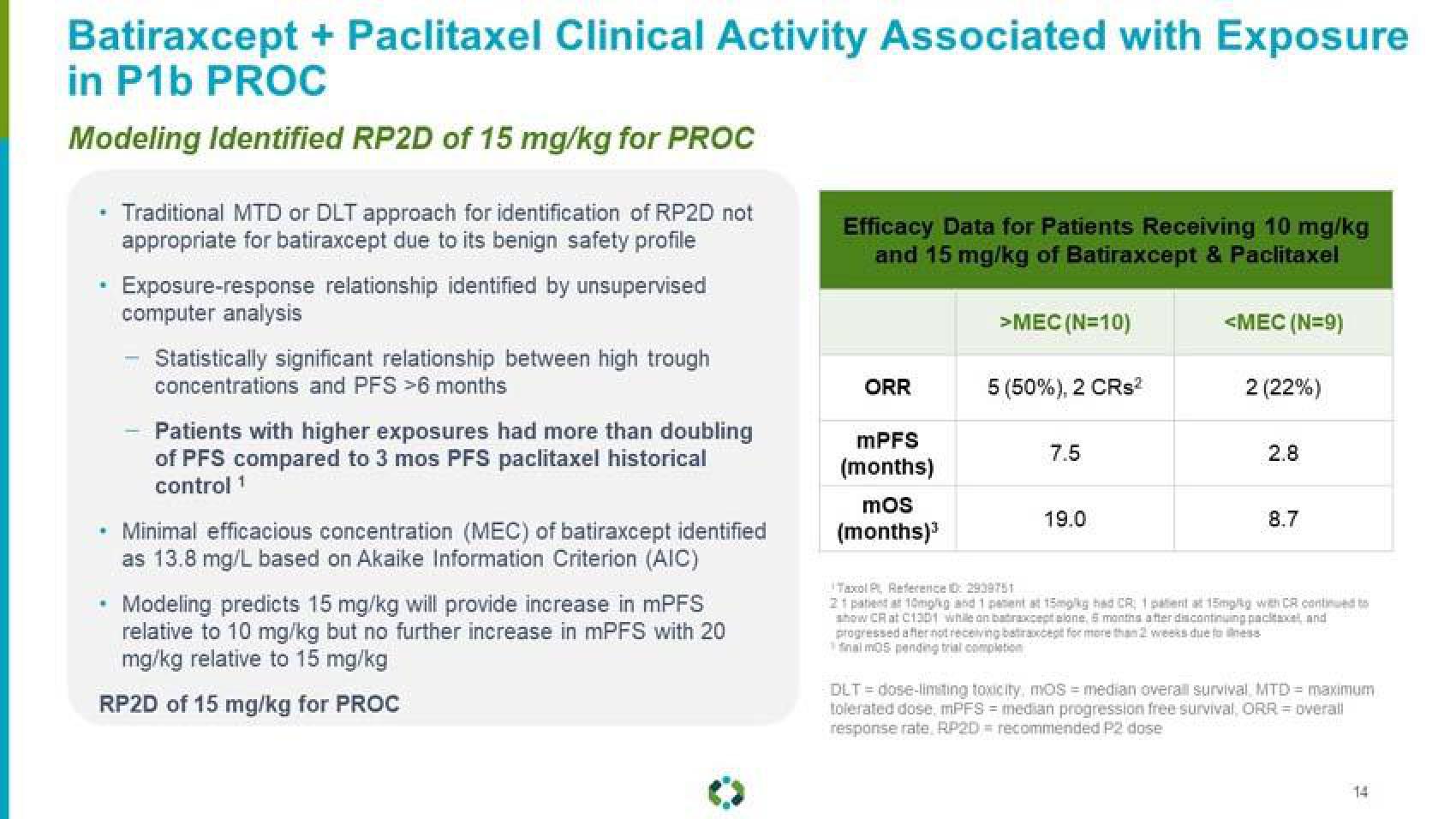
Batiraxcept + Paclitaxel Clinical Activity Associated with Exposure
in P1b PROC
Modeling Identified RP2D of 15 mg/kg for PROC
Traditional MTD or DLT approach for identification of RP2D not
appropriate for batiraxcept due to its benign safety profile
Exposure-response relationship identified by unsupervised
computer analysis
Statistically significant relationship between high trough
concentrations and PFS >6 months
Patients with higher exposures had more than doubling
of PFS compared to 3 mos PFS paclitaxel historical
control 1
Minimal efficacious concentration (MEC) of batiraxcept identified
as 13.8 mg/L based on Akaike Information Criterion (AIC)
Modeling predicts 15 mg/kg will provide increase in mPFS
relative to 10 mg/kg but no further increase in mPFS with 20
mg/kg relative to 15 mg/kg
RP2D of 15 mg/kg for PROC
Efficacy Data for Patients Receiving 10 mg/kg
and 15 mg/kg of Batiraxcept & Paclitaxel
>MEC (N=10)
5 (50%), 2 CRs²
ORR
mPFS
(months)
mos
(months)³
7.5
19.0
<MEC (N=9)
2 (22%)
2.8
8.7
Taxol PL Reference ID: 2939751
21 patient 10mplig and 1 patent at 15mg/kg had CR, 1 patent at 15mpg with CR continued to
show CR at C1301 while on braxcept alone, 5 months after discontinuing pace, and
progressed after not receiving betraxcept for more than 2 weeks due to ness
final mos pending trial completion
DLT= dose-limiting toxicity, mos median overall survival MTD= maximum
tolerated dose, mPFS = median progression free survival, ORR = overall
response rate. RP2D recommended P2 dose
14View entire presentation