ATAI Investor Presentation Deck
CADSS Score²
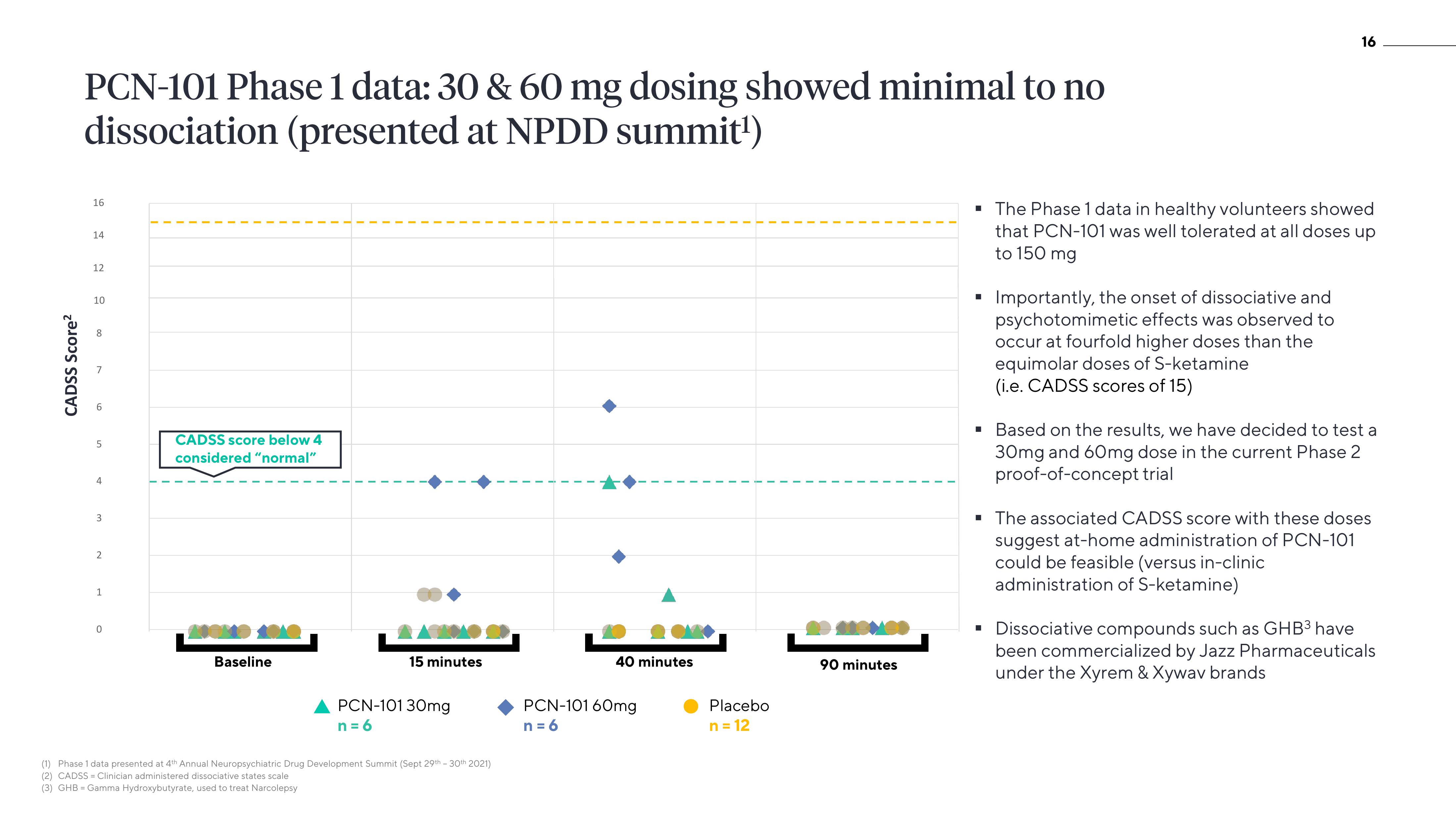
PCN-101 Phase 1 data: 30 & 60 mg dosing showed minimal to no
dissociation (presented at NPDD summit¹)
16
14
12
10
5
4
3
2
1
0
CADSS score below 4
considered "normal"
Baseline
15 minutes
PCN-101 30mg
n=6
(1) Phase 1 data presented at 4th Annual Neuropsychiatric Drug Development Summit (Sept 29th - 30th 2021)
(2) CADSS = Clinician administered dissociative states scale
(3) GHB = Gamma Hydroxybutyrate, used to treat Narcolepsy
40 minutes
PCN-101 60mg
n = 6
Placebo
n = 12
90 minutes
▪ The Phase 1 data in healthy volunteers showed
that PCN-101 was well tolerated at all doses up
to 150 mg
■
16
Importantly, the onset of dissociative and
psychotomimetic effects was observed to
occur at fourfold higher doses than the
equimolar doses of S-ketamine
(i.e. CADSS scores of 15)
▪ Based on the results, we have decided to test a
30mg and 60mg dose in the current Phase 2
proof-of-concept trial
▪ The associated CADSS score with these doses
suggest at-home administration of PCN-101
could be feasible (versus in-clinic
administration of S-ketamine)
▪ Dissociative compounds such as GHB3 have
been commercialized by Jazz Pharmaceuticals
under the Xyrem & Xywav brandsView entire presentation