BioAtla Investor Presentation Deck
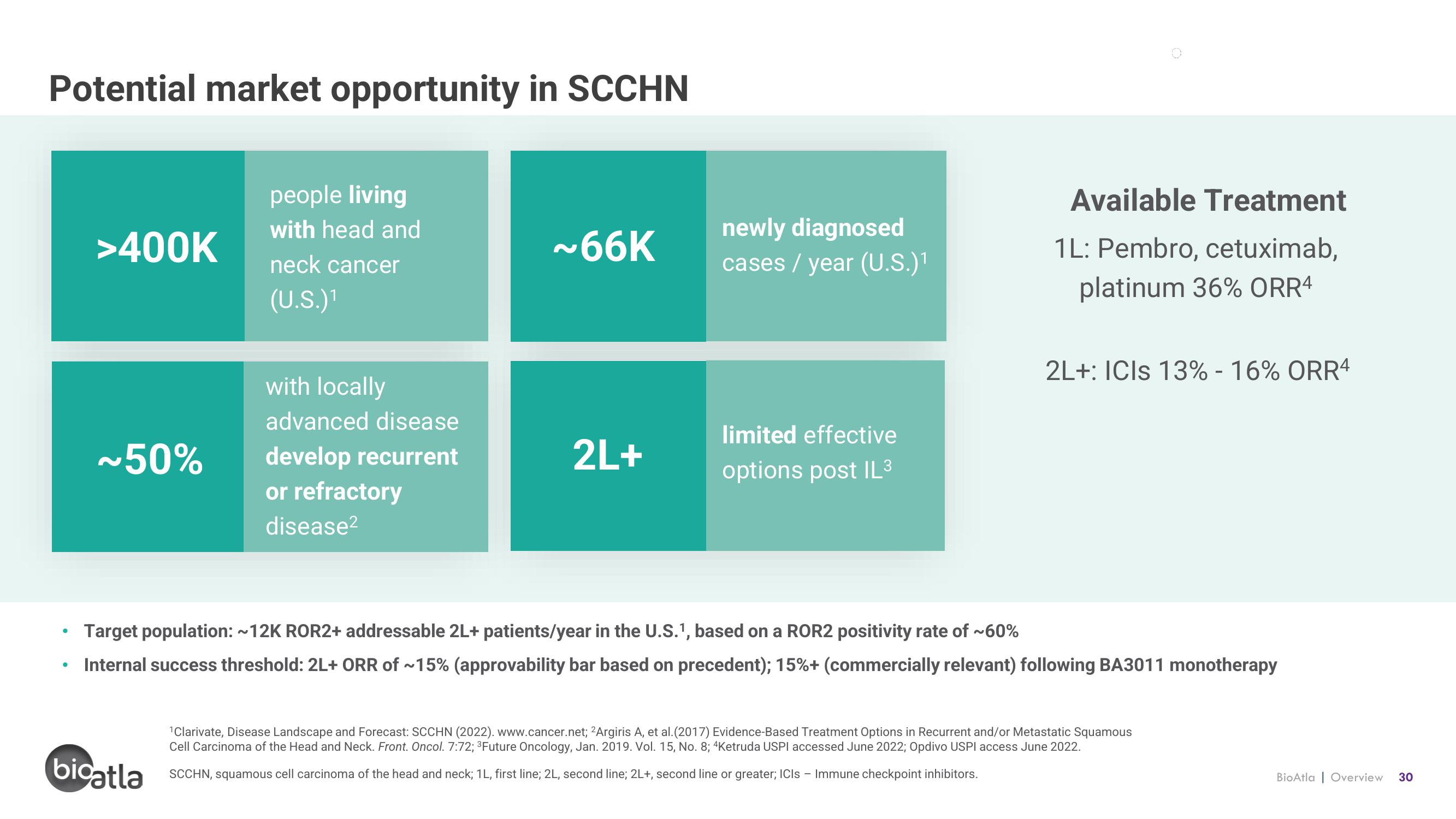
Potential market opportunity in SCCHN
●
>400K
~50%
people living
with head and
bicatla
neck cancer
(U.S.)¹1
with locally
advanced disease
develop recurrent
or refractory
disease²
~66K
2L+
newly diagnosed
cases / year (U.S.)¹
limited effective
options post IL³
Available Treatment
1L: Pembro, cetuximab,
platinum 36% ORR4
2L+: ICIS 13% -16% ORR4
Target population: ~12K ROR2+ addressable 2L+ patients/year in the U.S.1, based on a ROR2 positivity rate of ~60%
Internal success threshold: 2L+ ORR of ~15% (approvability bar based on precedent); 15% + (commercially relevant) following BA3011 monotherapy
¹Clarivate, Disease Landscape and Forecast: SCCHN (2022). www.cancer.net; 2Argiris A, et al. (2017) Evidence-Based Treatment Options in Recurrent and/or Metastatic Squamous
Cell Carcinoma of the Head and Neck. Front. Oncol. 7:72; ³Future Oncology, Jan. 2019. Vol. 15, No. 8; 4Ketruda USPI accessed June 2022; Opdivo USPI access June 2022.
SCCHN, squamous cell carcinoma of the head and neck; 1L, first line; 2L, second line; 2L+, second line or greater; ICIS - Immune checkpoint inhibitors.
BioAtla| Overview 30View entire presentation