Certara Investor Day Presentation Deck
Growing regulatory adoption and support
●
●
●
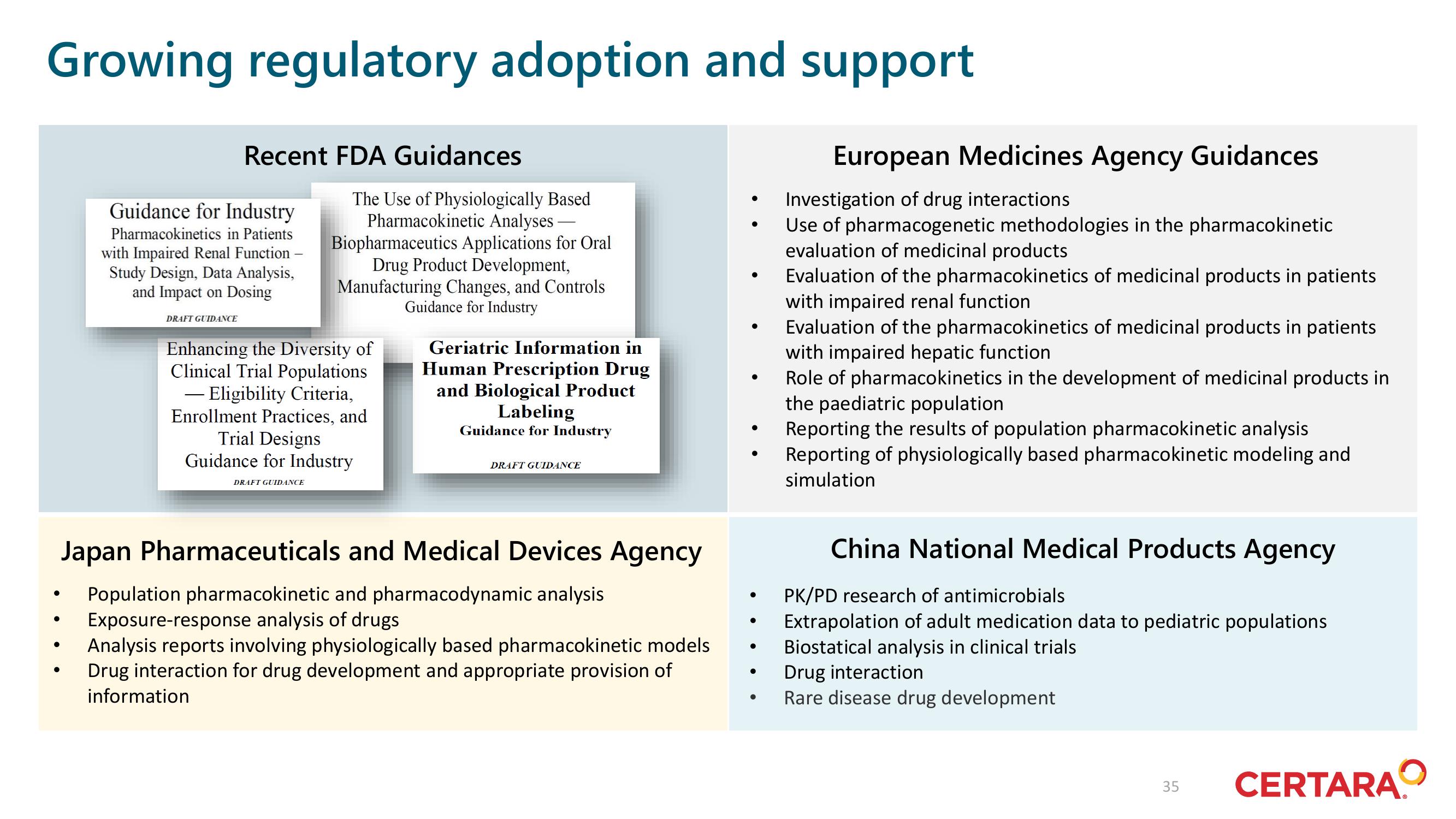
Recent FDA Guidances
Guidance for Industry
Pharmacokinetics in Patients
with Impaired Renal Function
Study Design, Data Analysis,
and Impact on Dosing
DRAFT GUIDANCE
The Use of Physiologically Based
Pharmacokinetic Analyses
Biopharmaceutics Applications for Oral
Drug Product Development,
Manufacturing Changes, and Controls
Guidance for Industry
Enhancing the Diversity of
Clinical Trial Populations
- Eligibility Criteria,
Enrollment Practices, and
Trial Designs
Guidance for Industry
DRAFT GUIDANCE
Japan Pharmaceuticals and Medical Devices Agency
Population pharmacokinetic and pharmacodynamic analysis
Exposure-response analysis of drugs
Analysis reports involving physiologically based pharmacokinetic models
Drug interaction for drug development and appropriate provision of
information
Geriatric Information in
Human Prescription Drug
and Biological Product
Labeling
Guidance for Industry
DRAFT GUIDANCE
●
●
●
●
●
●
●
●
European Medicines Agency Guidances
Investigation of drug interactions
Use of pharmacogenetic methodologies in the pharmacokinetic
evaluation of medicinal products
Evaluation of the pharmacokinetics of medicinal products in patients
with impaired renal function
Evaluation of the pharmacokinetics of medicinal products in patients
with impaired hepatic function
Role of pharmacokinetics in the development of medicinal products in
the paediatric population
Reporting the results of population pharmacokinetic analysis
Reporting of physiologically based pharmacokinetic modeling and
simulation
China National Medical Products Agency
PK/PD research of antimicrobials
Extrapolation of adult medication data to pediatric populations
Biostatical analysis in clinical trials
Drug interaction
Rare disease drug development
35
CERTARAView entire presentation