Certara Investor Day Presentation Deck
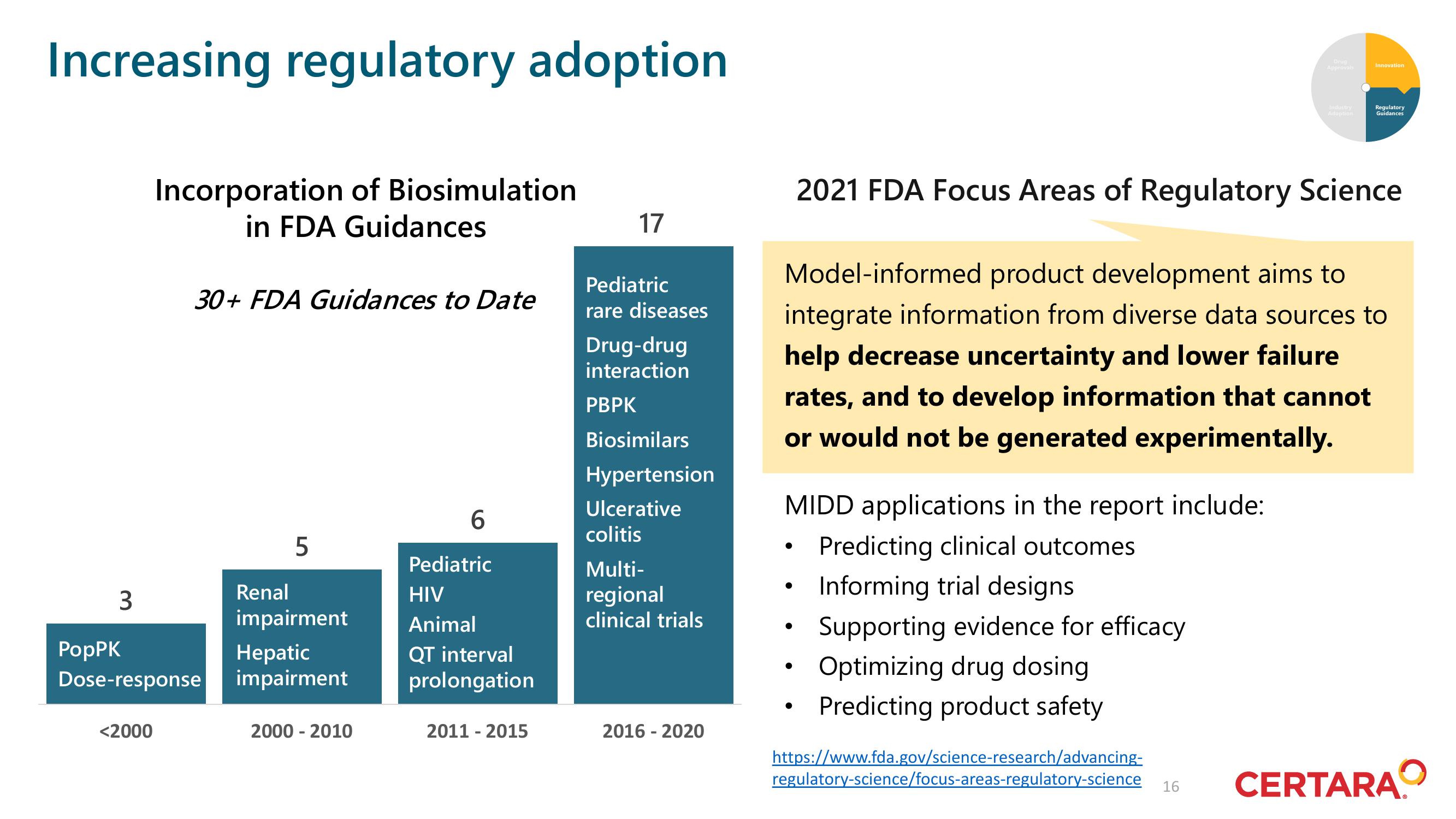
Increasing regulatory adoption
3
Incorporation of Biosimulation
in FDA Guidances
<2000
30+ FDA Guidances to Date
PopPK
Dose-response
5
Renal
impairment
Hepatic
impairment
2000 - 2010
6
Pediatric
HIV
Animal
QT interval
prolongation
2011-2015
17
Pediatric
rare diseases
Drug-drug
interaction
PBPK
Biosimilars
Hypertension
Ulcerative
colitis
Multi-
regional
clinical trials
2016-2020
MIDD applications in the report include:
Predicting clinical outcomes
Informing trial designs.
●
●
2021 FDA Focus Areas of Regulatory Science
Model-informed product development aims to
integrate information from diverse data sources to
help decrease uncertainty and lower failure
rates, and to develop information that cannot
or would not be generated experimentally.
Supporting evidence for efficacy
Optimizing drug dosing
Predicting product safety
Drug
Approvals
https://www.fda.gov/science-research/advancing-
regulatory-science/focus-areas-regulatory-science 16
Adoption
Innovation
Regulatory
Guidances
CERTARAView entire presentation