BioAtla IPO Presentation Deck
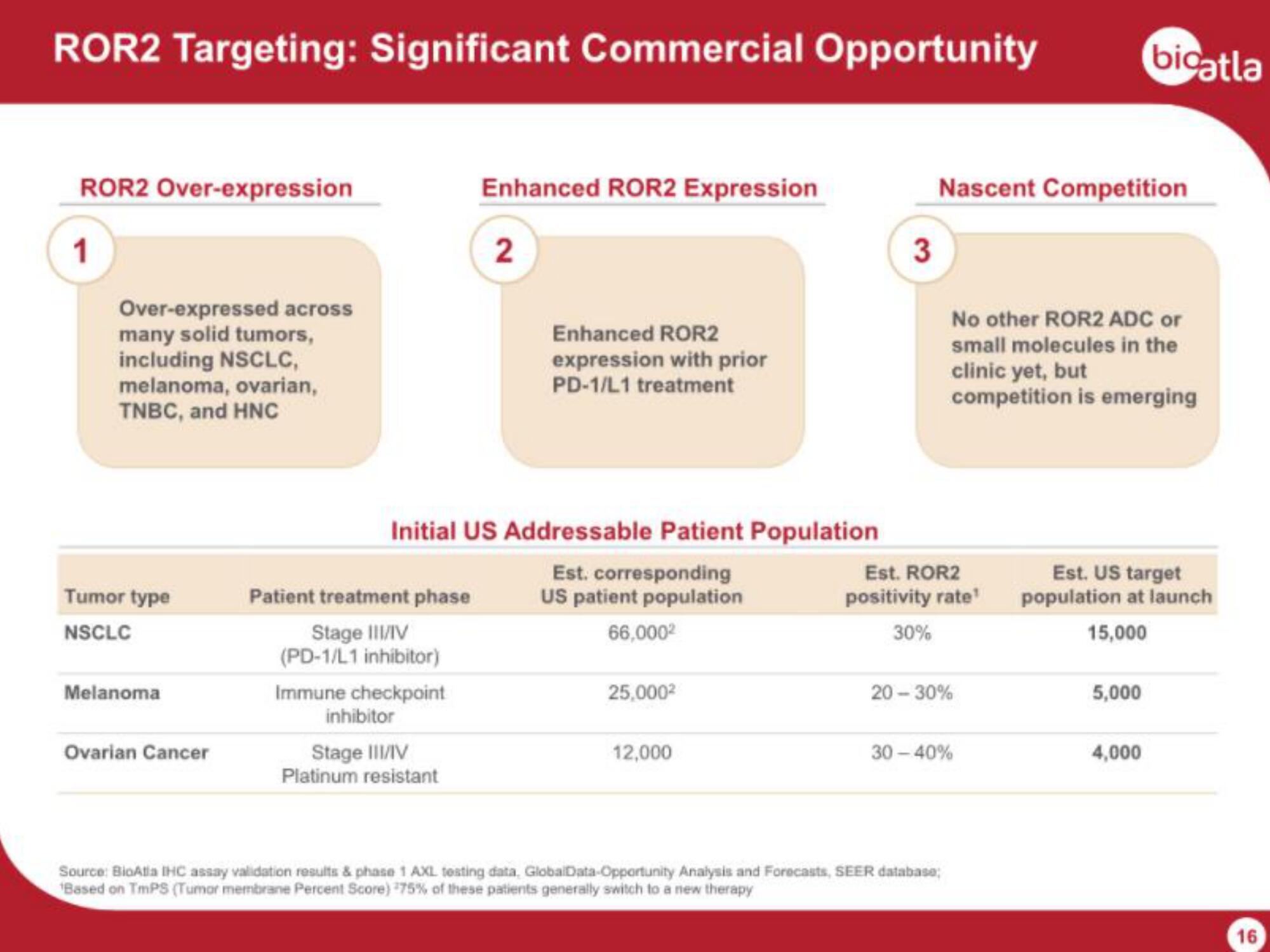
ROR2 Targeting: Significant Commercial Opportunity
ROR2 Over-expression
1
Over-expressed across
many solid tumors,
including NSCLC,
melanoma, ovarian,
TNBC, and HNC
Tumor type
NSCLC
Melanoma
Ovarian Cancer
Enhanced ROR2 Expression
2
Patient treatment phase
Stage III/IV
(PD-1/L1 inhibitor)
Immune checkpoint
inhibitor
Stage III/IV
Platinum resistant
Enhanced ROR2
expression with prior
PD-1/L1 treatment
Initial US Addressable Patient Population
Est. corresponding
US patient population
66,000²
25,000²
12,000
3
Nascent Competition
Est. ROR2
positivity rate¹
30%
No other ROR2 ADC or
small molecules in the
clinic yet, but
competition is emerging
20-30%
30-40%
Source: BioAtia IHC assay validation results & phase 1 AXL testing data, GlobaiData-Opportunity Analysis and Forecasts, SEER database;
Based on TmPS (Tumor membrane Percent Score) 75% of these patients generally switch to a new therapy
bicatla
Est. US target
population at launch
15,000
5,000
4,000
16View entire presentation