Immix Biopharma Investor Presentation Deck
Relapsed/Refractory Multiple Myeloma - Key Inclusion Criteria
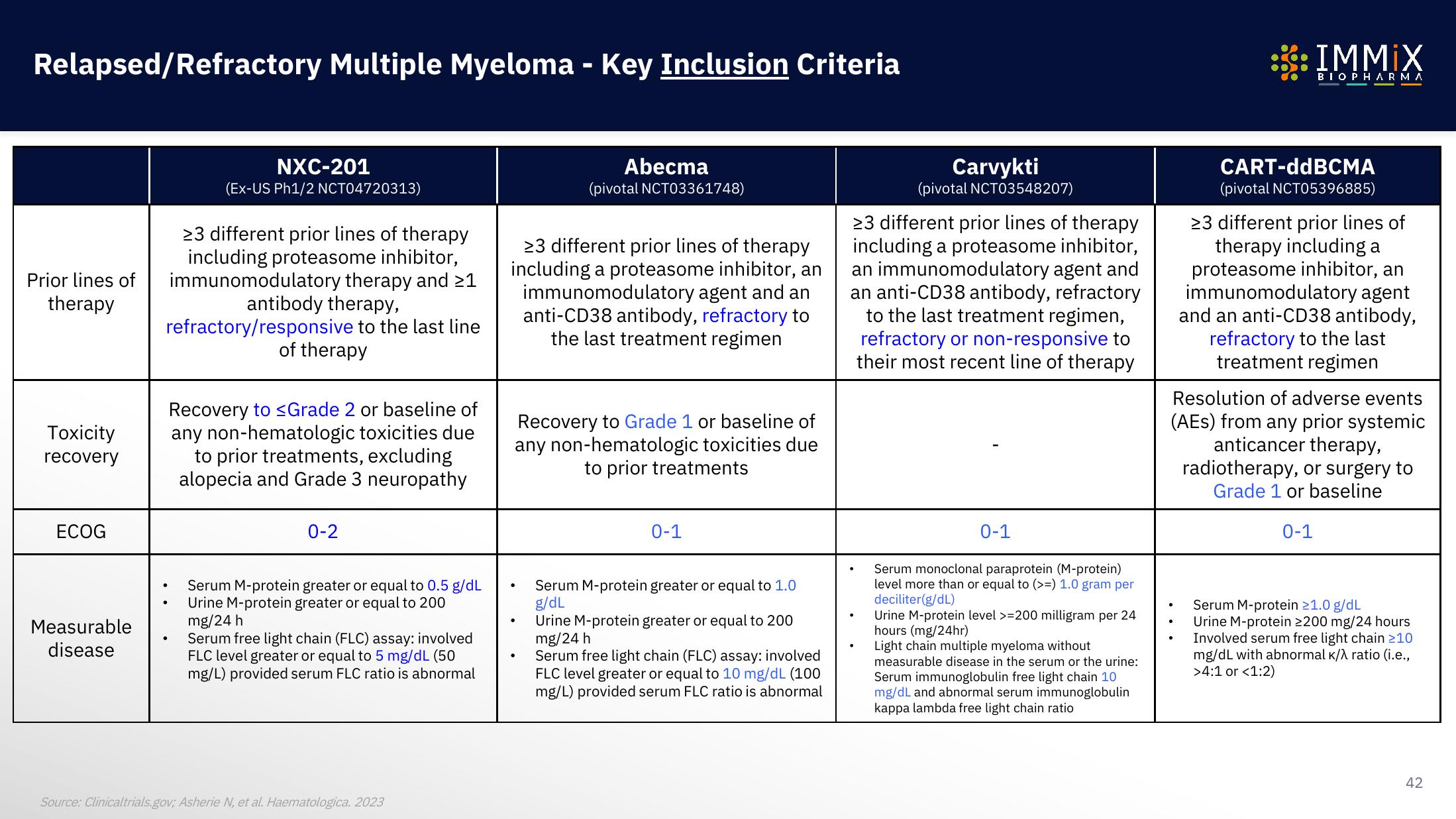
Prior lines of
therapy
Toxicity
recovery
ECOG
Measurable
disease
NXC-201
(Ex-US Ph1/2 NCT04720313)
>3 different prior lines of therapy
including proteasome inhibitor,
immunomodulatory therapy and >1
antibody therapy,
refractory/responsive to the last line
of therapy
Recovery to ≤Grade 2 or baseline of
any non-hematologic toxicities due
to prior treatments, excluding
alopecia and Grade 3 neuropathy
.
0-2
Serum M-protein greater or equal to 0.5 g/dL
Urine M-protein greater or equal to 200
mg/24 h
Serum free light chain (FLC) assay: involved
FLC level greater or equal to 5 mg/dL (50
mg/L) provided serum FLC ratio is abnormal
Source: Clinical trials.gov; Asherie N, et al. Haematologica. 2023
>3 different prior lines of therapy
including a proteasome inhibitor, an
immunomodulatory agent and an
anti-CD38 antibody, refractory to
the last treatment regimen
Abecma
(pivotal NCT03361748)
Recovery to Grade 1 or baseline of
any non-hematologic toxicities due
to prior treatments
.
.
0-1
Serum M-protein greater or equal to 1.0
g/dL
Urine M-protein greater or equal to 200
mg/24 h
Serum free light chain (FLC) assay: involved
FLC level greater or equal to 10 mg/dL (100
mg/L) provided serum FLC ratio is abnormal
Carvykti
(pivotal NCT03548207)
23 different prior lines of therapy
including a proteasome inhibitor,
an immunomodulatory agent and
an anti-CD38 antibody, refractory
to the last treatment regimen,
refractory or non-responsive to
their most recent line of therapy
0-1
Serum monoclonal paraprotein (M-protein)
level more than or equal to (>=) 1.0 gram per
deciliter (g/dL)
Urine M-protein level >=200 milligram per 24
hours (mg/24hr)
Light chain multiple myeloma without
measurable disease in the serum or the urine:
Serum immunoglobulin free light chain 10
mg/dL and abnormal serum immunoglobulin
kappa lambda free light chain ratio
●●●
S
IMMIX
BIOPHARMA
CART-ddBCMA
(pivotal NCT05396885)
23 different prior lines of
therapy including a
proteasome inhibitor, an
immunomodulatory agent
and an anti-CD38 antibody,
refractory to the last
treatment regimen
Resolution of adverse events
(AES) from any prior systemic
anticancer therapy,
radiotherapy, or surgery to
Grade 1 or baseline
0-1
Serum M-protein 21.0 g/dL
Urine M-protein 2200 mg/24 hours
Involved serum free light chain ≥10
mg/dL with abnormal K/λ ratio (i.e.,
>4:1 or <1:2)
42View entire presentation