BioAtla Investor Presentation Deck
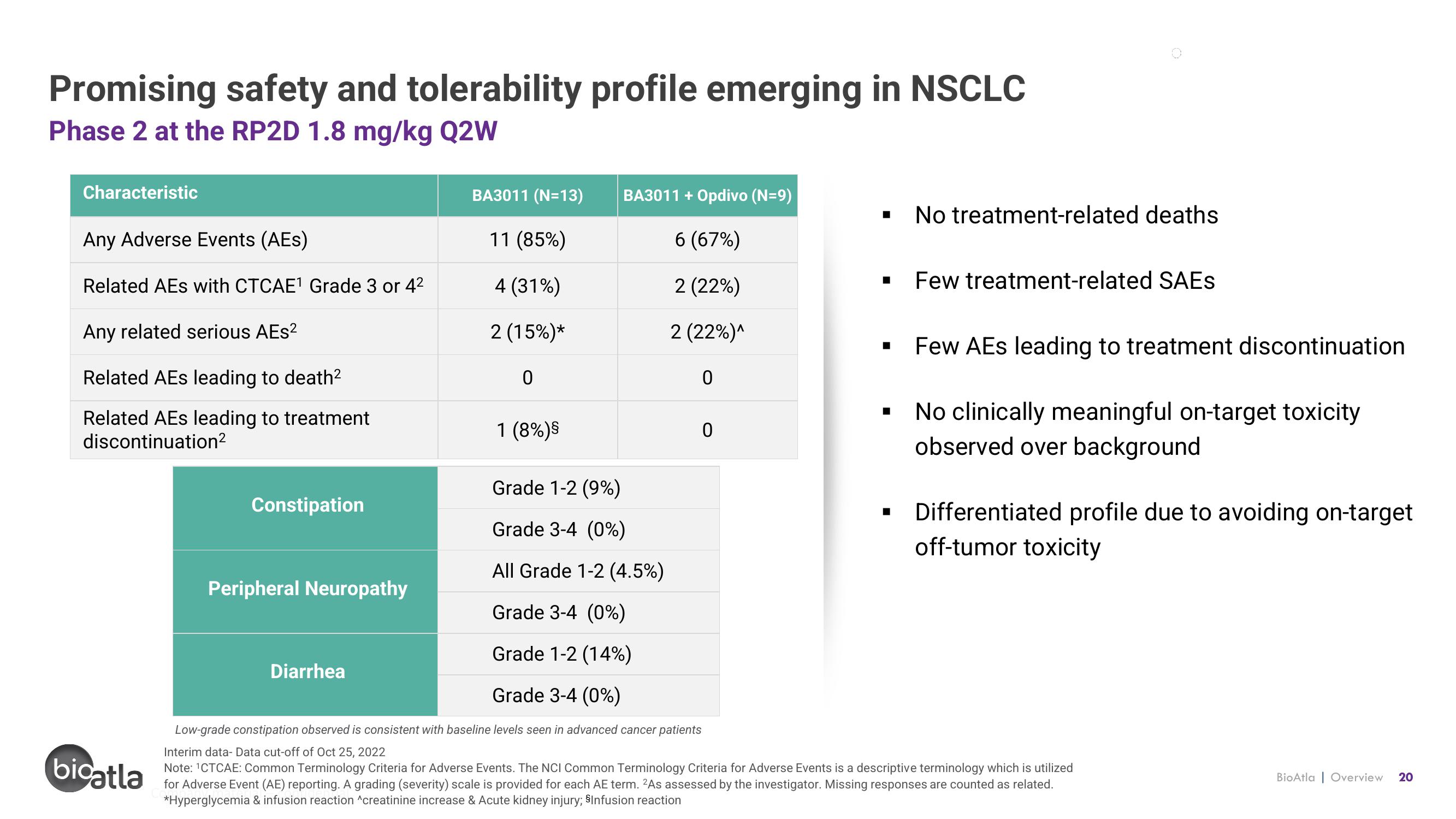
Promising safety and tolerability profile emerging in NSCLC
Phase 2 at the RP2D 1.8 mg/kg Q2W
Characteristic
Any Adverse Events (AES)
Related AEs with CTCAE¹ Grade 3 or 4²
Any related serious AEs²
Related AEs leading to death²
Related AEs leading to treatment
discontinuation²
bicatla
Constipation
Peripheral Neuropathy
BA3011 (N=13)
11 (85%)
4 (31%)
2 (15%)*
Diarrhea
0
1 (8%) ⁹
BA3011 + Opdivo (N=9)
6 (67%)
2 (22%)
2 (22%)^
0
0
■
■
■
■
No treatment-related deaths
Few treatment-related SAEs
Grade 1-2 (9%)
Grade 3-4 (0%)
All Grade 1-2 (4.5%)
Grade 3-4 (0%)
Grade 1-2 (14%)
Grade 3-4 (0%)
Low-grade constipation observed is consistent with baseline levels seen in advanced cancer patients
Interim data- Data cut-off of Oct 25, 2022
Note: ¹CTCAE: Common Terminology Criteria for Adverse Events. The NCI Common Terminology Criteria for Adverse Events is a descriptive terminology which is utilized
for Adverse Event (AE) reporting. A grading (severity) scale is provided for each AE term. 2As assessed by the investigator. Missing responses are counted as related.
*Hyperglycemia & infusion reaction ^creatinine increase & Acute kidney injury; §Infusion reaction
Few AEs leading to treatment discontinuation
No clinically meaningful on-target toxicity
observed over background
Differentiated profile due to avoiding on-target
off-tumor toxicity
BioAtla| Overview 20View entire presentation